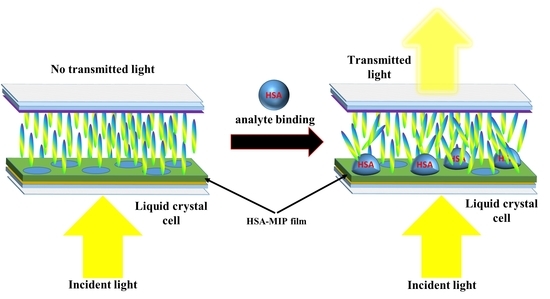
Protein Determination with Molecularly Imprinted Polymer Recognition Combined with Birefringence Liquid Crystal Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Procedures
2.3.1. Synthesis of Functional Monomers
2.3.2. Synthesis of the HSA-Templated Molecularly Imprinted Polymer (MIP-HSA) Film and the Control Non-Imprinted Polymer (NIP) Film
2.3.3. SPR Determination of HSA
2.3.4. Procedure of the HSA Determination Using Liquid Crystal Optical Cells
3. Results and Discussion
3.1. MIP and NIP Film Deposition and Characterization
3.2. SPR Sensor Preparation and Testing
3.3. LC Sensor Preparation and Testing
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, V.K.; Skaife, J.J.; Dubrovsky, T.B.; Abbott, N.L. Optical amplification of ligand-receptor binding using liquid crystals. Science 1998, 279, 2077–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.R.; Abbott, N.L. Rubbed films of functionalized bovine serum albumin as substrates for the imaging of protein-receptor interactions using liquid crystals. Adv. Mater. 2001, 13, 1445–1449. [Google Scholar] [CrossRef]
- Xue, C.Y.; Yang, K.L. Dark-to-bright optical responses of liquid crystals supported on solid surfaces decorated with proteins. Langmuir 2008, 24, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Clare, B.H.; Abbott, N.L. Orientations of nentatic liquid crystals on surfaces presenting controlled densities of peptides: Amplification of protein-peptide binding events. Langmuir 2005, 21, 6451–6461. [Google Scholar] [CrossRef]
- Das, D.; Sidiq, S.; Pal, S.K. A simple quantitative method to study protein-lipopolysaccharide interactions by using liquid crystals. Chem. Phys. Chem. 2015, 16, 753–760. [Google Scholar] [CrossRef]
- Su, H.W.; Lee, Y.H.; Lee, M.J.; Hsu, Y.C.; Lee, W. Label-free immunodetection of the cancer biomarker CA125 using high-Delta n liquid crystals. J. Biomed. Opt. 2014, 19, 077006. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Yang, K.L. Detection and quantification of DNA adsorbed on solid surfaces by using liquid crystals. Langmuir 2010, 26, 1427–1430. [Google Scholar] [CrossRef]
- Price, A.D.; Schwartz, D.K. DNA hybridization-induced reorientation of liquid crystal anchoring at the nematic liquid crystal/aqueous interface. J. Am. Chem. Soc. 2008, 130, 8188–8194. [Google Scholar] [CrossRef]
- Tan, H.; Yang, S.Y.; Shen, G.L.; Yu, R.Q.; Wu, Z.Y. Signal-enhanced liquid-crystal DNA biosensors based on enzymatic metal deposition. Angew. Chem. Int. Edit. 2010, 49, 8608–8611. [Google Scholar] [CrossRef]
- Yang, S.Y.; Liu, Y.M.; Tan, H.; Wu, C.; Wu, Z.Y.; Shen, G.L.; Yu, R.Q. Gold nanoparticle based signal enhancement liquid crystal biosensors for DNA hybridization assays. Chem. Commun. 2012, 48, 2861–2863. [Google Scholar] [CrossRef]
- Yang, S.Y.; Wu, C.; Tan, H.; Wu, Y.; Liao, S.Z.; Wu, Z.Y.; Shen, G.L.; Yu, R.Q. Label-free liquid crystal biosensor based on specific oligonucleotide probes for heavy metal ions. Anal. Chem. 2013, 85, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Lee, W. Liquid crystal-based capacitive, electro-optical and dielectric biosensors for protein quantitation. Liq. Cryst. 2019, 1145–1153. [Google Scholar] [CrossRef]
- Peters, J.T. All About Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Curry, S.; Mandelkow, H.; Brick, P.; Franks, N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat. Struct. Biol. 1998, 5, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.H.; Winyard, P.J.; Koletzko, S.; Wehner, B.; Cheema, H.A.; Risdon, R.A.; Phillips, A.D.; Meadows, N.; Klein, N.J.; Walker-Smith, J.A. Congenital enterocyte heparan sulphate deficiency with massive albumin loss, secretory diarrhoea, and malnutrition. Lancet 1996, 347, 1299–1301. [Google Scholar] [CrossRef]
- Danesh, J.; Collins, R.; Appleby, P.; Peto, R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease—Meta-analyses of prospective studies. J. Am. Med. Assoc. 1998, 279, 1477–1482. [Google Scholar] [CrossRef]
- Greipp, P.R.; San Miguel, J.; Durie, B.G.M.; Crowley, J.J.; Barlogie, B.; Blade, J.; Boccadoro, M.; Child, J.A.; Harousseau, J.L.; Kyle, R.A.; et al. International staging system for multiple myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Hoogenberg, K.; Sluiter, W.J.; Dullaart, R.P. Effect of growth hormone and insulin-like growth factor I on urinary albumin excretion: Studies in acromegaly and growth hormone deficiency. Acta Endocrinol. (Cph.) 1993, 129, 151–157. [Google Scholar] [CrossRef]
- Rodkey, F.L. Direct spectrophotometric determination of albumin in human serum. Clin. Chem. 1965, 11, 478–487. [Google Scholar] [CrossRef]
- Volkova, K.D.; Kovalska, V.B.; Losytskyy, M.Y.; Reis, L.V.; Santos, P.F.; Almeida, P.; Lynch, D.E.; Yarmoluk, S.M. Aza-substituted squaraines for the fluorescent detection of albumins. Dye. Pigment. 2011, 90, 41–47. [Google Scholar] [CrossRef]
- Smith, S.E.; Williams, J.M.; Ando, S.; Koide, K. Time-insensitive fluorescent sensor for human serum albumin and its unusual red shift. Anal. Chem. 2014, 86, 2332–2336. [Google Scholar] [CrossRef]
- Caballero, D.; Martinez, E.; Bausells, J.; Errachid, A.; Samitier, J. Impedimetric immunosensor for human serum albumin detection on a direct aldehyde functionalized silicon nitride surface. Anal. Chim. Acta 2012, 720, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, N.M.; Peppas, N.A. Molecularly imprinted polymers with specific recognition for macromolecules and proteins. Prog. Polym. Sci. 2008, 33, 271–288. [Google Scholar] [CrossRef]
- Bossi, A.; Bonini, F.; Turner, A.P.F.; Piletsky, S.A. Molecularly imprinted polymers for the recognition of proteins: The state of the art. Biosens. Bioelectron. 2007, 22, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Kryscio, D.R.; Peppas, N.A. Critical review and perspective of macromolecularly imprinted polymers. Acta Biomater. 2012, 8, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Verheyen, E.; Schillemans, J.P.; van Wijk, M.; Demeniex, M.A.; Hennink, W.E.; van Nostrum, C.F. Challenges for the effective molecular imprinting of proteins. Biomaterials 2011, 32, 3008–3020. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.G.; Zhang, L.H.; Liang, Z.; Zhang, Y.K. Protein-imprinted materials: Rational design, application and challenges. Anal. Bioanal. Chem. 2012, 403, 2173–2183. [Google Scholar] [CrossRef]
- Cieplak, M.; Kutner, W. Artificial biosensors: How can molecular imprinting mimic biorecognition? Trends Biotechnol. 2016, 34, 922–941. [Google Scholar] [CrossRef]
- Iskierko, Z.; Sharma, P.S.; Bartold, K.; Pietrzyk-Le, A.; Noworyta, K.; Kutner, W. Molecularly imprinted polymers for separating and sensing of macromolecular compounds and microorganisms. Biotechnol. Adv. 2016, 34, 30–46. [Google Scholar] [CrossRef]
- Zhang, L.-P.; Wang, X.-L.; Pang, Q.-Q.; Huang, Y.-P.; Tang, L.; Chen, M.; Liu, Z.-S. Solvent-responsive floating liquid crystalline-molecularly imprinted polymers for gastroretentive controlled drug release system. Int. J. Pharmaceut. 2017, 532, 365–373. [Google Scholar] [CrossRef]
- Binet, C.; Bourrier, D.; Dilhan, M.; Esteve, D.; Ferrere, S.; Garrigue, J.-C.; Granier, H.; Lattes, A.; Gue, A.-M.; Mauzaca, M.; et al. First approach to the use of liquid crystal elastomers for chemical sensors. Talanta 2006, 69, 757–762. [Google Scholar] [CrossRef]
- Cieplak, M.; Węgłowski, R.; Iskierko, Z.; Węgłowska, D.; Sharma, P.S.; Noworyta, K.; D’Souza, F.; Kutner, W. Optical chemosensor for protein determination with molecularly imprinted polymer recognition and birefringence liquid crystal detection. In Proceedings of the 8th International Workshop on Surface Modification for Chemical and Biochemical Sensing, Żelechów, Poland, 3–7 November 2017; p. 230. [Google Scholar]
- Cieplak, M.; Szwabinska, K.; Sosnowska, M.; Chandra, B.K.C.; Borowicz, P.; Noworyta, K.; D’Souza, F.; Kutner, W. Selective electrochemical sensing of human serum albumin by semi-covalent molecular imprinting. Biosens. Bioelectron. 2015, 74, 960–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.T.; Wang, Q.H.; Kempe, M.D.; Kornfield, J.A. Perdeuterated cyanobiphenyl liquid crystals for infrared applications. J. Appl. Phys. 2002, 92, 7146–7148. [Google Scholar] [CrossRef] [Green Version]
- Huynh, T.P.; Bikram, K.C.C.; Lisowski, W.; D’Souza, F.; Kutner, W. Molecularly imprinted polymer of bis(2,2′-bithienyl)methanes for selective determination of adrenaline. Bioelectrochemistry 2013, 93, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.P.; Sosnowska, M.; Sobczak, J.W.; Kc, C.B.; Nesterov, V.N.; D’Souza, F.; Kutner, W. Simultaneous chronoamperometry and piezoelectric microgravimetry determination of nitroaromatic explosives using molecularly imprinted thiophene polymers. Anal. Chem. 2013, 85, 8361–8368. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieplak, M.; Węgłowski, R.; Iskierko, Z.; Węgłowska, D.; Sharma, P.S.; Noworyta, K.R.; D’Souza, F.; Kutner, W. Protein Determination with Molecularly Imprinted Polymer Recognition Combined with Birefringence Liquid Crystal Detection. Sensors 2020, 20, 4692. https://doi.org/10.3390/s20174692
Cieplak M, Węgłowski R, Iskierko Z, Węgłowska D, Sharma PS, Noworyta KR, D’Souza F, Kutner W. Protein Determination with Molecularly Imprinted Polymer Recognition Combined with Birefringence Liquid Crystal Detection. Sensors. 2020; 20(17):4692. https://doi.org/10.3390/s20174692
Chicago/Turabian StyleCieplak, Maciej, Rafał Węgłowski, Zofia Iskierko, Dorota Węgłowska, Piyush S. Sharma, Krzysztof R. Noworyta, Francis D’Souza, and Wlodzimierz Kutner. 2020. "Protein Determination with Molecularly Imprinted Polymer Recognition Combined with Birefringence Liquid Crystal Detection" Sensors 20, no. 17: 4692. https://doi.org/10.3390/s20174692