Regional-Scale Monitoring of Wheat Stripe Rust Using Remote Sensing and Geographical Detectors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Survey and Data Collection
2.1.1. Study Area and Field-Experimental-Data Acquirement
2.1.2. Meteorological Data and Preprocessing
2.1.3. Remote Sensing Data and Preprocessing
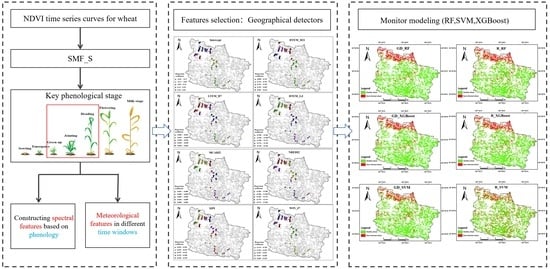
2.2. Remote Sensing Monitoring of Wheat Stripe Rust at a Regional Scale Based on Geographical Detectors
2.2.1. Extraction of Key Phenological Stages in Wheat
2.2.2. Multi-Source Feature Construction for Wheat-Stripe-Rust Monitoring
2.2.3. Feature Selection for Disease and Pest Monitoring Using Geographical Detectors
2.2.4. Monitoring Modeling
3. Results
3.1. Wheat Key-Phenological-Stage Extraction Results
3.2. Feature Importance Analysis
3.3. Monitoring Feature Selection
3.4. Accuracy Validation of Monitoring Results
4. Discussion
4.1. Analysis of Spatial-Distribution Differences of Wheat Stripe Rust in the Study Area
4.2. Analysis of the Performance of Spectral Features and Meteorological Features
4.3. Discussion for Next Steps for Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X. Pathogens Which Threaten Food Security: Puccinia Striiformis, the Wheat Stripe Rust Pathogen. Food Secur. 2020, 12, 239–251. [Google Scholar] [CrossRef]
- Wellings, C.R. Global Status of Stripe Rust: A Review of Historical and Current Threats. Euphytica 2011, 179, 129–141. [Google Scholar] [CrossRef]
- Beddow, J.M.; Pardey, P.G.; Chai, Y.; Hurley, T.M.; Kriticos, D.J.; Braun, H.-J.; Park, R.F.; Cuddy, W.S.; Yonow, T. Research Investment Implications of Shifts in the Global Geography of Wheat Stripe Rust. Nat. Plants 2015, 1, 15132. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kang, Z.; Ma, Z.; Xu, S.; Jin, S.; Jiang, Y. Integrated management of wheat stripe rust caused by Puccinia striiformis f. sp. tritici in China. Sci. Agric. 2013, 46, 4254–4262. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y.; Huang, W.; Dong, Y.; Ma, H.; Wu, K.; Guo, A. Combining Random Forest and XGBoost Methods in Detecting Early and Mid-Term Winter Wheat Stripe Rust Using Canopy Level Hyperspectral Measurements. Agriculture 2022, 12, 74. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, L.; Wang, J.; Luo, J.; Du, S.; Huang, W. Research Progress of Crop Diseases and Pests Monitoring Based on Remote Sensing. Trans. Chin. Soc. Agric. Eng. 2012, 28, 1–11. [Google Scholar]
- Berger, K.; Machwitz, M.; Kycko, M.; Kefauver, S.C.; Van Wittenberghe, S.; Gerhards, M.; Verrelst, J.; Atzberger, C.; van der Tol, C.; Damm, A.; et al. Multi-Sensor Spectral Synergies for Crop Stress Detection and Monitoring in the Optical Domain: A Review. Remote Sens. Environ. 2022, 280, 113198. [Google Scholar] [CrossRef]
- Das, S.; Biswas, A.; Vimalkumar, C.; Sinha, P. Deep Learning Analysis of Rice Blast Disease Using Remote Sensing Images. IEEE Geosci. Remote Sens. Lett. 2023, 20, 2500905. [Google Scholar] [CrossRef]
- Cotrozzi, L. Spectroscopic Detection of Forest Diseases: A Review (1970–2020). J. For. Res. 2022, 33, 21–38. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Steiner, U.; Hillnhütter, C.; Dehne, H.-W.; Oerke, E.-C. Hyperspectral Imaging for Small-Scale Analysis of Symptoms Caused by Different Sugar Beet Diseases. Plant Methods 2012, 8, 3. [Google Scholar] [CrossRef]
- Calderón, R.; Navas-Cortés, J.A.; Lucena, C.; Zarco-Tejada, P.J. High-Resolution Airborne Hyperspectral and Thermal Imagery for Early Detection of Verticillium Wilt of Olive Using Fluorescence, Temperature and Narrow-Band Spectral Indices. Remote Sens. Environ. 2013, 139, 231–245. [Google Scholar] [CrossRef]
- Huang, W.; Shi, Y.; Dong, Y.; Ye, H.; Wu, M.; Cui, B.; Liu, L. Progress and Prospects of Crop Diseases and Pests Monitoring by Remote Sensing. Smart Agric. 2019, 1, 1–11. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, W.; Cui, X.; Shi, Y.; Liu, L. New Spectral Index for Detecting Wheat Yellow Rust Using Sentinel-2 Multispectral Imagery. Sensors 2018, 18, 868. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Krishnan, P.; Singh, V.K.; Das, B. Estimation of Yellow Rust Severity in Wheat Using Visible and Thermal Imaging Coupled with Machine Learning Models. Geocarto Int. 2023, 38, 2160831. [Google Scholar] [CrossRef]
- Huang, W.; Lu, J.; Ye, H.; Kong, W.; Mortimer, A.H.; Shi, Y. Quantitative Identification of Crop Disease and Nitrogen-Water Stress in Winter Wheat Using Continuous Wavelet Analysis. Int. J. Agric. Biol. Eng. 2018, 11, 145–152. [Google Scholar] [CrossRef]
- Jing, X.; Du, K.; Duan, W.; Zou, Q.; Zhao, T.; Li, B.; Ye, Q.; Yan, L. Quantifying the Effects of Stripe Rust Disease on Wheat Canopy Spectrum Based on Eliminating Non-Physiological Stresses. Crop J. 2022, 10, 1284–1291. [Google Scholar] [CrossRef]
- Guo, A.; Huang, W.; Dong, Y.; Ye, H.; Ma, H.; Liu, B.; Wu, W.; Ren, Y.; Ruan, C.; Geng, Y. Wheat Yellow Rust Detection Using UAV-Based Hyperspectral Technology. Remote Sens. 2021, 13, 123. [Google Scholar] [CrossRef]
- Zheng, Q.; Ye, H.; Huang, W.; Dong, Y.; Jiang, H.; Wang, C.; Li, D.; Wang, L.; Chen, S. Integrating Spectral Information and Meteorological Data to Monitor Wheat Yellow Rust at a Regional Scale: A Case Study. Remote Sens. 2021, 13, 278. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Yuan, L.; Yang, G.; Chen, L.; Zhao, C. Using Satellite Multispectral Imagery for Damage Mapping of Armyworm (Spodoptera frugiperda) in Maize at a Regional Scale. Pest Manag. Sci. 2016, 72, 335–348. [Google Scholar] [CrossRef]
- Du, X.; Li, Q.; Shang, J.; Liu, J.; Qian, B.; Jing, Q.; Dong, T.; Fan, D.; Wang, H.; Zhao, L.; et al. Detecting Advanced Stages of Winter Wheat Yellow Rust and Aphid Infection Using RapidEye Data in North China Plain. GISci. Remote Sens. 2019, 56, 1093–1113. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, W.; Ye, H.; Ruan, C.; Xing, N.; Geng, Y.; Dong, Y.; Peng, D. Partial Least Square Discriminant Analysis Based on Normalized Two-Stage Vegetation Indices for Mapping Damage from Rice Diseases Using PlanetScope Datasets. Sensors 2018, 18, 1901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Pu, R.; Gonzalez-Moreno, P.; Yuan, L.; Wu, K.; Huang, W. Monitoring Plant Diseases and Pests through Remote Sensing Technology: A Review. Comput. Electron. Agric. 2019, 165, 104943. [Google Scholar] [CrossRef]
- Anderegg, J.; Hund, A.; Karisto, P.; Mikaberidze, A. In-Field Detection and Quantification of Septoria Tritici Blotch in Diverse Wheat Germplasm Using Spectral–Temporal Features. Front. Plant Sci. 2019, 10, 1355. [Google Scholar] [CrossRef]
- Pryzant, R.; Ermon, S.; Lobell, D. Monitoring Ethiopian Wheat Fungus With Satellite Imagery and Deep Feature Learning. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition Workshops (CVPRW), Honolulu, HI, USA, 21–26 July 2017; pp. 1524–1532. [Google Scholar]
- Gongora-Canul, C.; Salgado, J.D.; Singh, D.; Cruz, A.P.; Cotrozzi, L.; Couture, J.; Rivadeneira, M.G.; Cruppe, G.; Valent, B.; Todd, T.; et al. Temporal Dynamics of Wheat Blast Epidemics and Disease Measurements Using Multispectral Imagery. Phytopathology 2020, 110, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhong, Y.; Hu, X.; Wei, L.; Zhang, L. A Robust Spectral-Spatial Approach to Identifying Heterogeneous Crops Using Remote Sensing Imagery with High Spectral and Spatial Resolutions. Remote Sens. Environ. 2020, 239, 111605. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Z.-W.; Lin, S.-T.; Li, S.; Fang, Z.-B. Spatial point pattern analysis of pine wilt disease occurrence and its influence factors. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2022, 33, 2530–2538. [Google Scholar] [CrossRef]
- Zhang, J. Regional Forcasting of Rice Disease Prevalence Based on Multi-Source Spatio-Temporal Information. Master’s Thesis, Hangzhou Dianzi University, Hangzhou, China, 2022. [Google Scholar] [CrossRef]
- Yuan, L.; Bao, Z.; Zhang, H.; Zhang, Y.; Liang, X. Habitat Monitoring to Evaluate Crop Disease and Pest Distributions Based on Multi-Source Satellite Remote Sensing Imagery. Optik 2017, 145, 66–73. [Google Scholar] [CrossRef]
- Backoulou, G.F.; Elliott, N.C.; Giles, K.; Phoofolo, M.; Catana, V.; Mirik, M.; Michels, J. Spatially Discriminating Russian Wheat Aphid Induced Plant Stress from Other Wheat Stressing Factors. Comput. Electron. Agric. 2011, 78, 123–129. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Bouwman, J.J. 11—Epidemiology in Europe. In Diseases, Distribution, Epidemiology, and Control; Roelfs, A.P., Bushnell, W.R., Eds.; Academic Press: New York, NY, USA, 1985; pp. 329–369. ISBN 978-0-12-148402-6. [Google Scholar]
- Luo, C.; Ma, L.; Zhu, J.; Guo, Z.; Dong, K.; Dong, Y. Effects of Nitrogen and Intercropping on the Occurrence of Wheat Powdery Mildew and Stripe Rust and the Relationship With Crop Yield. Front. Plant Sci. 2021, 12, 637393. [Google Scholar] [CrossRef]
- Ruan, C.; Dong, Y.; Huang, W.; Huang, L.; Ye, H.; Ma, H.; Guo, A.; Sun, R. Integrating Remote Sensing and Meteorological Data to Predict Wheat Stripe Rust. Remote Sens. 2022, 14, 1221. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, P.; Sun, H.; Zhang, S.; Li, L. Assimilation of Leaf Area Index and Surface Soil Moisture With the CERES-Wheat Model for Winter Wheat Yield Estimation Using a Particle Filter Algorithm. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2017, 10, 1303–1316. [Google Scholar] [CrossRef]
- Ruan, C.; Dong, Y.; Huang, W.; Huang, L.; Ye, H.; Ma, H.; Guo, A.; Ren, Y. Prediction of Wheat Stripe Rust Occurrence with Time Series Sentinel-2 Images. Agriculture 2021, 11, 1079. [Google Scholar] [CrossRef]
- Hutchinson, M.F. A New Procedure for Gridding Elevation and Stream Line Data with Automatic Removal of Spurious Pits. J. Hydrol. 1989, 106, 211–232. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Yang, F.; Fu, C.; Teng, F.; Gao, J. Early Recognition of Winter Wheat Area Based on GF-1 Satellite. Trans. Chin. Soc. Agric. Eng. 2015, 31, 194–201. [Google Scholar]
- Liu, L.; Cao, R.; Chen, J.; Shen, M.; Wang, S.; Zhou, J.; He, B. Detecting Crop Phenology from Vegetation Index Time-Series Data by Improved Shape Model Fitting in Each Phenological Stage. Remote Sens. Environ. 2022, 277, 113060. [Google Scholar] [CrossRef]
- Chen, J.; Jönsson, P.; Tamura, M.; Gu, Z.; Matsushita, B.; Eklundh, L. A Simple Method for Reconstructing a High-Quality NDVI Time-Series Data Set Based on the Savitzky–Golay Filter. Remote Sens. Environ. 2004, 91, 332–344. [Google Scholar] [CrossRef]
- Yuan, L. Identification and Differentiation of Wheat Diseases and Insects with Multi-Source and Multi-Scale Remote Sensing Data. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2015. [Google Scholar]
- Ceccato, P.; Flasse, S.; Tarantola, S.; Jacquemoud, S.; Grégoire, J.-M. Detecting Vegetation Leaf Water Content Using Reflectance in the Optical Domain. Remote Sens. Environ. 2001, 77, 22–33. [Google Scholar] [CrossRef]
- Galvão, L.S.; Formaggio, A.R.; Tisot, D.A. Discrimination of Sugarcane Varieties in Southeastern Brazil with EO-1 Hyperion Data. Remote Sens. Environ. 2005, 94, 523–534. [Google Scholar] [CrossRef]
- Fensholt, R.; Sandholt, I. Derivation of a Shortwave Infrared Water Stress Index from MODIS Near- and Shortwave Infrared Data in a Semiarid Environment. Remote Sens. Environ. 2003, 87, 111–121. [Google Scholar] [CrossRef]
- Louhaichi, M.; Borman, M.M.; Johnson, D.E. Spatially Located Platform and Aerial Photography for Documentation of Grazing Impacts on Wheat. Geocarto Int. 2008, 16, 65–70. [Google Scholar] [CrossRef]
- Kanemasu, E.T. Seasonal Canopy Reflectance Patterns of Wheat, Sorghum, and Soybean. Remote Sens. Environ. 1974, 3, 43–47. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-Based Plant Height from Crop Surface Models, Visible, and near Infrared Vegetation Indices for Biomass Monitoring in Barley. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Penuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the Radiometric and Biophysical Performance of the MODIS Vegetation Indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Woebbecke, D.M.; Meyer, G.E.; Von Bargen, K.; Mortensen, D.A. Color indices for weed identification under various soil, residue, and lighting conditions. Trans ASABE 1995, 38, 259–269. [Google Scholar] [CrossRef]
- Kaufman, Y.J.; Tanre, D. Atmospherically Resistant Vegetation Index (ARVI) for EOS-MODIS. IEEE Trans. Geosci. Remote Sens. 1992, 30, 261–270. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of vegetation indices and a modified simple ratio for boreal applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of Soil-Adjusted Vegetation Indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR Absorbed by Vegetation from Bidirectional Reflectance Measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of Leaf-Area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Quantitative Estimation of Chlorophyll-a Using Reflectance Spectra: Experiments with Autumn Chestnut and Maple Leaves. J. Photochem. Photobiol. B 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Fernández-Manso, A.; Fernández-Manso, O.; Quintano, C. SENTINEL-2A Red-Edge Spectral Indices Suitability for Discriminating Burn Severity. Int. J. Appl. Earth Obs. Geoinf. 2016, 50, 170–175. [Google Scholar] [CrossRef]
- Danson, F.M.; Plummer, S.E. Red-Edge Response to Forest Leaf Area Index. Int. J. Remote Sens. 1995, 16, 183–188. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing Prediction Power and Stability of Broadband and Hyperspectral Vegetation Indices for Estimation of Green Leaf Area Index and Canopy Chlorophyll Density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Christakos, G.; Liao, Y.; Zhang, T.; Gu, X.; Zheng, X. Geographical Detectors-Based Health Risk Assessment and Its Application in the Neural Tube Defects Study of the Heshun Region, China. Int. J. Geogr. Inf. Sci. 2010, 24, 107–127. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Ge, Y.; Xu, C. An Optimal Parameters-Based Geographical Detector Model Enhances Geographic Characteristics of Explanatory Variables for Spatial Heterogeneity Analysis: Cases with Different Types of Spatial Data. GISci. Remote Sens. 2020, 57, 593–610. [Google Scholar] [CrossRef]
- Guyon, I.; Weston, J.; Barnhill, S.; Vapnik, V. Gene Selection for Cancer Classification Using Support Vector Machines. Mach. Learn. 2002, 46, 389–422. [Google Scholar] [CrossRef]
- Granitto, P.M.; Furlanello, C.; Biasioli, F.; Gasperi, F. Recursive Feature Elimination with Random Forest for PTR-MS Analysis of Agroindustrial Products. Chemom. Intell. Lab. Syst. 2006, 83, 83–90. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Rumpf, T.; Welke, P.; Dehne, H.-W.; Plümer, L.; Steiner, U.; Oerke, E.-C. Development of Spectral Indices for Detecting and Identifying Plant Diseases. Remote Sens. Environ. 2013, 128, 21–30. [Google Scholar] [CrossRef]
- Huang, W.; Guan, Q.; Luo, J.; Zhang, J.; Zhao, J.; Liang, D.; Huang, L.; Zhang, D. New Optimized Spectral Indices for Identifying and Monitoring Winter Wheat Diseases. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 2516–2524. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, T.; Chen, W.; Sankaran, S. Non-Invasive Evaluation of Ascochyta Blight Disease Severity in Chickpea Using Field Asymmetric Ion Mobility Spectrometry and Hyperspectral Imaging Techniques. Crop Prot. 2023, 165, 106163. [Google Scholar] [CrossRef]
- Kononenko, I. Estimating Attributes: Analysis and Extensions of RELIEF. In Proceedings of the Machine Learning: ECML-94; Bergadano, F., De Raedt, L., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 171–182. [Google Scholar]
- Ashourloo, D.; Nematollahi, H.; Huete, A.; Aghighi, H.; Azadbakht, M.; Shahrabi, H.S.; Goodarzdashti, S. A New Phenology-Based Method for Mapping Wheat and Barley Using Time-Series of Sentinel-2 Images. Remote Sens. Environ. 2022, 280, 113206. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Y.; Ata-Ul-Karim, S.T.; Ge, Q.; Li, X.; Xiao, J. Integrating Climate and Satellite Remote Sensing Data for Predicting County-Level Wheat Yield in China Using Machine Learning Methods. Int. J. Appl. Earth Obs. Geoinf. 2022, 111, 102861. [Google Scholar] [CrossRef]
- Chen, D.; Shi, Y.; Huang, W.; Zhang, J.; Wu, K. Mapping Wheat Rust Based on High Spatial Resolution Satellite Imagery. Comput. Electron. Agric. 2018, 152, 109–116. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Ham, J.; Chen, Y.; Crawford, M.M.; Ghosh, J. Investigation of the Random Forest Framework for Classification of Hyperspectral Data. IEEE Trans. Geosci. Remote Sens. 2005, 43, 492–501. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13 August 2016; ACM: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Liao, C.; Wang, J.; Shan, B.; Shang, J.; Dong, T.; He, Y. Near Real-Time Detection and Forecasting of within-Field Phenology of Winter Wheat and Corn Using Sentinel-2 Time-Series Data. ISPRS J. Photogramm. Remote Sens. 2023, 196, 105–119. [Google Scholar] [CrossRef]
- Scholthof, K.-B.G. The Disease Triangle: Pathogens, the Environment and Society. Nat. Rev. Microbiol. 2007, 5, 152–156. [Google Scholar] [CrossRef]
- Rong, F.; Di, B.; Jiang, Y. Numerical simulation of the long distance atmospheric transport of wheat stripe rust spores: A case study. Acta Ecol. Sin. 2009, 29, 3952–3959. [Google Scholar]
- Naseri, B.; Sasani, S. Cultivar, Planting Date and Weather Linked to Wheat Leaf Rust Development. Cereal Res. Commun. 2020, 48, 203–210. [Google Scholar] [CrossRef]
- Pan, A. Exploration of the Epidemic Patterns of Wheat Stripe Rust in the Autumn Seedling Stage in the Winter-Spring Wheat Intercropping Zone. Inform. Agric. Sci. Tech. 2013, 21, 1003–6997. [Google Scholar]
- Grabow, B.S.; Shah, D.A.; DeWolf, E.D. Environmental Conditions Associated with Stripe Rust in Kansas Winter Wheat. Plant Dis. 2016, 100, 2306–2312. [Google Scholar] [CrossRef]
- Wu, Q.; Feng, H.; Li, H.; Wan, D.; Jia, Q.; Li, M.; Liang, H. Effect of Puccinia striiformins West infection on cyanideresistant respiration and metabolism of reactive oxygen in wheat. Acta Phytopathol. Sin. 2006, 36, 49–56. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhao, J.; Wu, J.; Zhan, G.; Han, D.; Kang, Z. Wheat stripe rust and integration of sustainable control strategies in china. Front. Agric. Sci. Eng. 2022, 9, 37. [Google Scholar] [CrossRef]
- El Jarroudi, M.; Kouadio, L.; Bock, C.H.; El Jarroudi, M.; Junk, J.; Pasquali, M.; Maraite, H.; Delfosse, P. A Threshold-Based Weather Model for Predicting Stripe Rust Infection in Winter Wheat. Plant Dis. 2017, 101, 693–703. [Google Scholar] [CrossRef]
- Stubbs, R.W. Stripe Rust: The Cereal Rusts II: Diseases, Distribution, Epidemiology, and Control; Roelfs, A.P., Bushnell, W.R., Eds.; Academic Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Cotrozzi, L.; Couture, J.J. Hyperspectral Assessment of Plant Responses to Multi-Stress Environments: Prospects for Managing Protected Agrosystems. Plants People Planet 2020, 2, 244–258. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Peron, R.; Tuinstra, M.R.; Mickelbart, M.V.; Couture, J.J. Spectral Phenotyping of Physiological and Anatomical Leaf Traits Related with Maize Water Status. Plant Physiol. 2020, 184, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, K.; Xing, X.; Guo, H.; Zhang, W.; Luo, Q.; Gao, S.; Huang, Z.; Li, H.; Li, X.; et al. On spatial effects in geographical analysis. J. Geogr. 2023, 78, 517–531. [Google Scholar] [CrossRef]











| Correlation | Vegetation Indices | Formula |
|---|---|---|
| Water content | Moisture Stress index, MSI [41] | |
| Disease Water Stress Index, DSWI [42] | ||
| Shortwave Infrared Water Stress Index, SIWSI [43] | ||
| Pigment content | Green Leaf Index, GLI [44] | |
| Greenness Ratio Vegetation index, GRVI [45] | ||
| Modified Chlorophyll-Absorption-Ratio Index, MCARIn [46] | ||
| Red–Green–Blue Vegetation Index, RGBVI [47] | ||
| Structure-Independent Pigment Index, SIPI [48] | ||
| Normalized Difference Vegetation Index, NDVI [49] | ||
| Green-Normalized Difference Vegetation Index, GNDVI [50] | ||
| Excessive Green Index, ExG [51] | ||
| Vegetation coverage | Atmospherically Resistant Vegetation Index, ARVI [52] | |
| Difference Vegetation Index, DVI [53] | ||
| Enhanced Vegetation Index, EVI [50] | ||
| Modified Simple Ratio Index, MSR [54] | ||
| Optimized Soil-Adjusted Vegetation Index, OSAVI [55] | ||
| Renormalized Difference Vegetation Index, RDVI [56] | ||
| Simple Ratio Index, SR [57] | ||
| Stress status | Normalized Difference Vegetation Index Red Edge, NDVIreln [49] | |
| Normalized Red-edge 1 Index, NREDI1 [58] | ||
| Normalized Red-edge 2 Index, NREDI2 [58] | ||
| Normalized Red-edge 3 Index, NREDI3 [58] | ||
| Plant Senescence Reflectance Index, PSRIn [59] | ||
| Red-edge Disease Stress Index, REDSI [13] | ||
| Red-edge Inflection Point, REIP [60] | ||
| Triangular Vegetation Index, TVI [61] | ||
| Band |
| Method | Number | Feature |
|---|---|---|
| Geographical detectors | 11 | HTEM_H21, LTEM_H7, HTEM_GJ, HTEM_J21, PRE_GJ, RHU_H15, WIN_J7, WIN_G21, NREDI2, SIPI, MCARI2 |
| ReliefF | 6 | SSD_H21, PRE_H15, HTEM_J21, PRE_GJ, SIPI, REDSI |
| Method | Parameters of RF | Parameters of XGBoost | Parameters of SVM | |||
|---|---|---|---|---|---|---|
| n_Estimators | max_Depth | n_Estimators | max_Depth | C | Gamma | |
| Geographic Detector | 23 | 5 | 14 | 2 | 2 | 0.05 |
| ReliefF | 15 | 3 | 11 | 3 | 1 | 0.05 |
| Method | Model | Healthy | Infected | Sum | UA | OA | Kappa | |
|---|---|---|---|---|---|---|---|---|
| Geographic Detectors | RF | Healthy | 45 | 5 | 50 | 90.0% | 87.2% | 0.743 |
| Infected | 7 | 37 | 44 | 84.9% | ||||
| Sum | 52 | 42 | 94 | |||||
| PA | 86.5% | 88.1% | ||||||
| XGBoost | Healthy | 40 | 10 | 50 | 80.0% | 80.9% | 0.614 | |
| Infected | 8 | 36 | 44 | 81.8% | ||||
| Sum | 48 | 46 | 94 | |||||
| PA | 83.3% | 78.3% | ||||||
| SVM | Healthy | 40 | 10 | 50 | 80.0% | 74.5% | 0.484 | |
| Infected | 14 | 30 | 44 | 68.2% | ||||
| Sum | 54 | 40 | 94 | |||||
| PA | 74.1% | 75.0% | ||||||
| ReliefF | RF | Healthy | 43 | 7 | 50 | 86.0% | 84.0% | 0.679 |
| Infected | 8 | 36 | 44 | 81.8% | ||||
| Sum | 51 | 43 | 94 | |||||
| PA | 84.3% | 83.7% | ||||||
| XGBoost | Healthy | 42 | 8 | 50 | 84.0% | 78.7% | 0.570 | |
| Infected | 12 | 32 | 44 | 72.7% | ||||
| Sum | 54 | 40 | 94 | |||||
| PA | 77.8% | 80.0% | ||||||
| SVM | Healthy | 39 | 11 | 50 | 78.0% | 70.2% | 0.397 | |
| Infected | 17 | 27 | 44 | 61.4% | ||||
| Sum | 56 | 38 | 94 | |||||
| PA | 69.6% | 71.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Dong, Y.; Huang, W.; Ruan, C.; Guo, J. Regional-Scale Monitoring of Wheat Stripe Rust Using Remote Sensing and Geographical Detectors. Remote Sens. 2023, 15, 4631. https://doi.org/10.3390/rs15184631
Zhao M, Dong Y, Huang W, Ruan C, Guo J. Regional-Scale Monitoring of Wheat Stripe Rust Using Remote Sensing and Geographical Detectors. Remote Sensing. 2023; 15(18):4631. https://doi.org/10.3390/rs15184631
Chicago/Turabian StyleZhao, Mingxian, Yingying Dong, Wenjiang Huang, Chao Ruan, and Jing Guo. 2023. "Regional-Scale Monitoring of Wheat Stripe Rust Using Remote Sensing and Geographical Detectors" Remote Sensing 15, no. 18: 4631. https://doi.org/10.3390/rs15184631