Morphological and Physiological Screening to Predict Lettuce Biomass Production in Controlled Environment Agriculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growing Conditions
2.3. Projected Canopy Size Imaging
2.4. Diurnal Changes in Photochemistry
ΦPSII and ETR Light Response Curve
2.5. Harvest
2.6. Data Analysis
3. Results
3.1. Growth Differences among Cultivars
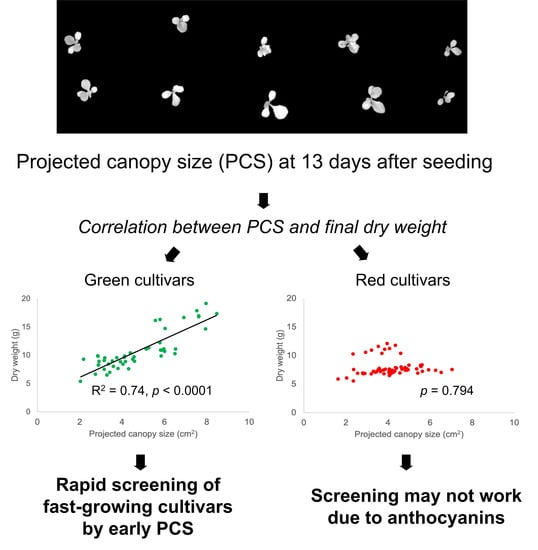
3.2. Biomass and Projected Canopy Size Were Correlated
3.3. ΦPSII and ETR Light Response Curves and Their Relationship to Biomass
3.4. Correlation between Biomass and Canopy ETR or Total Incident Light
3.5. Light Use Efficiency
4. Discussion
4.1. Quantifying Canopy Size Using CFI
4.2. PCS Screening for Fast Growth
4.3. Light Use Efficiency
4.4. Inhibitory Effect of Anthocyanins on LUE and Biomass Accumulation
4.5. No Correlation between Leaf ETR and Biomass
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pattison, P.M.; Tsao, J.Y.; Brainard, G.C.; Bugbee, B. LEDs for photons, physiology and food. Nature 2018, 563, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Van Iersel, M.W. Optimizing LED lighting in controlled environment agriculture. In Light Emitting Diodes for Agriculture: Smart Lighting; Gupta, S.D., Ed.; Springer: Singapore, 2017; pp. 59–80. [Google Scholar]
- Kozai, T. Smart Plant Factory: The Next Generation Indoor Vertical Farms; Springer: Singapore, 2018. [Google Scholar]
- Watson, R.T.; Boudreau, M.-C.; Van Iersel, M.W. Simulation of greenhouse energy use: An application of energy informatics. Energy Inform. 2018, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Gómez, C.; Currey, C.J.; Dickson, R.W.; Kim, H.-J.; Hernández, R.; Sabeh, N.C.; Raudales, R.E.; Brumfield, R.G.; Laury-Shaw, A.; Wilke, A.K.; et al. Controlled Environment Food Production for Urban Agriculture. HortScience 2019, 54, 1448–1458. [Google Scholar] [CrossRef]
- Folta, K.M. Breeding new varieties for controlled environments. Plant Biol (Stuttg) 2019, 21 (Suppl. 1), 6–12. [Google Scholar] [CrossRef]
- Goudriaan, J.; Monteith, J.L. A Mathematical Function for Crop Growth Based on Light Interception and Leaf Area Expansion. Ann. Bot. 1990, 66, 695–701. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef] [Green Version]
- McCree, K.J.; Troughton, J.H. Prediction of Growth Rate at Different Light Levels from Measured Photosynthesis and Respiration Rates. Plant Physiol. 1966, 41, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Li, C.; Paterson, A.H.; Jiang, Y.; Xu, R.; Robertson, J.S.; Snider, J.L.; Chee, P.W. In-field High Throughput Phenotyping and Cotton Plant Growth Analysis Using LiDAR. Front. Plant Sci. 2018, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Christensen, S.; Goudriaan, J. Deriving light interception and biomass from spectral reflectance ratio. Remote Sens. Environ. 1993, 43, 87–95. [Google Scholar] [CrossRef]
- Rosati, A. Estimating Canopy Light Interception and Absorption Using Leaf Mass Per Unit Leaf Area in Solanum melongena. Ann. Bot. 2001, 88, 101–109. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [Green Version]
- Müller, P.; Li, X.-P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Yang, W.; Duan, L.; Chen, G.; Xiong, L.; Liu, Q. Plant phenomics and high-throughput phenotyping: Accelerating rice functional genomics using multidisciplinary technologies. Curr. Opin. Plant Biol. 2013, 16, 180–187. [Google Scholar] [CrossRef]
- Barbagallo, R.P.; Oxborough, K.; Pallett, K.E.; Baker, N.R. Rapid, noninvasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant Physiol. 2003, 132, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Purcell, L.C. Soybean Canopy Coverage and Light Interception Measurements Using Digital Imagery. Crop Sci. 2000, 40, 834–837. [Google Scholar] [CrossRef]
- Nyakwende, E.; Paull, C.J.; Atherton, J.G. Non-destructive determination of leaf area in tomato plants using image processing. J. Hortic. Sci. 1997, 72, 255–262. [Google Scholar] [CrossRef]
- Chen, D.; Shi, R.; Pape, J.M.; Neumann, K.; Arend, D.; Graner, A.; Chen, M.; Klukas, C. Predicting plant biomass accumulation from image-derived parameters. Gigascience 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Makanza, R.; Zaman-Allah, M.; Cairns, J.; Magorokosho, C.; Tarekegne, A.; Olsen, M.; Prasanna, B. High-Throughput Phenotyping of Canopy Cover and Senescence in Maize Field Trials Using Aerial Digital Canopy Imaging. Remote Sens. 2018, 10, 330. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; CRC Press: London, UK, 2000; pp. 445–483. [Google Scholar]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu Rev. Plant Biol 2008, 59, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Jaramillo, A.A.; Duarte-Galvan, C.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Romero-Troncoso, R.D.J.; Guevara-Gonzalez, R.G.; Millan-Almaraz, J.R. Instrumentation in Developing Chlorophyll Fluorescence Biosensing: A Review. Sensors 2012, 12, 11853–11869. [Google Scholar] [CrossRef]
- Pedrós, R.; Moya, I.; Goulas, Y.; Jacquemoud, S. Chlorophyll fluorescence emission spectrum inside a leaf. Photochem. Photobiol. Sci. 2008, 7, 498. [Google Scholar] [CrossRef] [Green Version]
- Toda, S.; Takayam, K.; Kanoh, T.; Fujiuchi, N.; Takahashi, N.; Nishina, H. Measurement of daily stem elongtation with chlorophyll fluorescence imaging robot. Eco-Engineering 2020, 32, 15–21. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Weaver, G.; Van Iersel, M.W. Photochemical Characterization of Greenhouse-grown Lettuce (Lactuca sativa L. ‘Green Towers’) with Applications for Supplemental Lighting Control. HortScience 2019, 54, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Omasa, K.; Hosoi, F.; Konishi, A. 3D lidar imaging for detecting and understanding plant responses and canopy structure. J. Exp. Bot. 2007, 58, 881–898. [Google Scholar] [CrossRef] [Green Version]
- Gholz, H.L.; Vogel, S.A.; Cropper, W.P., Jr.; McKelvey, K.; Ewel, K.C.; Teskey, R.O.; Curran, P.J. Dynamics of Canopy Structure and Light Interception in Pinus Elliottii Stands, North Florida. Ecol. Monogr. 1991, 61, 33–51. [Google Scholar] [CrossRef] [Green Version]
- Geipel, J.; Link, J.; Claupein, W. Combined Spectral and Spatial Modeling of Corn Yield Based on Aerial Images and Crop Surface Models Acquired with an Unmanned Aircraft System. Remote Sens. 2014, 6, 10335–10355. [Google Scholar] [CrossRef] [Green Version]
- Jayalath, T.C.; Van Iersel, M.W. Canopy Size and Light Use Efficiency Explain Growth Differences between Lettuce and Mizuna in Vertical Farms. Plants 2021, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Klassen, S.P.; Ritchie, G.; Frantz, J.M.; Pinnock, D.; Bugbee, B. Real-Time Imaging of Ground Cover: Relationships with Radiation Capture, Canopy Photosynthesis, and Daily Growth Rate. In Imaging and Spectral Techniques: Applications to Precision Agriculture and Crop Physiology; ASA-CSSA-SSSA: Baltimore, MD, USA, 2001; pp. 1–14. [Google Scholar]
- Legendre, R.; Van Iersel, M.W. Supplemental Far-Red Light Stimulates Lettuce Growth: Disentangling Morphological and Physiological Effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.-H.; Park, S.H.; Han, X.Z.; Kim, H.-J. Image Processing Methods for Measurement of Lettuce Fresh Weight. J. Biosyst. Eng. 2015, 40, 89–93. [Google Scholar] [CrossRef] [Green Version]
- WüNsche, J.N.; Lakso, A.N. The Relationship Between Leaf Area and Light Interception by Spur and Extension Shoot Leaves and Apple Orchard Productivity. HortScience 2000, 35, 1202–1206. [Google Scholar] [CrossRef] [Green Version]
- Cabrera-Bosquet, L.; Fournier, C.; Brichet, N.; Welcker, C.; Suard, B.; Tardieu, F. High-throughput estimation of incident light, light interception and radiation-use efficiency of thousands of plants in a phenotyping platform. New Phytol 2016, 212, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Horton, P. Prospects for crop improvement through the genetic manipulation of photosynthesis: Morphological and biochemical aspects of light capture. J. Exp. Bot. 2000, 51, 475–485. [Google Scholar] [CrossRef]
- Wells, R. Soybean Growth Response to Plant Density: Relationships among Canopy Photosynthesis, Leaf Area, and Light Interception. Crop Sci. 1991, 31, 755–761. [Google Scholar] [CrossRef]
- Loomis, R.S.; Amthor, J.S. Yield Potential, Plant Assimilatory Capacity, and Metabolic Efficiencies. Crop Sci. 1999, 39, 1584–1596. [Google Scholar] [CrossRef] [Green Version]
- Slattery, R.A.; Ort, D.R. Perspectives on improving light distribution and light use efficiency in crop canopies. Plant Physiol. 2021, 185, 34–48. [Google Scholar] [CrossRef]
- Kage, H.; Stützel, H.; Alt, C. Predicting dry matter production of cauliflower (Brassica oleracea L. botrytis) under unstressed conditions: Part II. Comparison of light use efficiency and photosynthesis–respiration based modules. Sci. Hortic. 2001, 87, 171–190. [Google Scholar] [CrossRef]
- Van Iersel, M. Carbon use efficiency depends on growth respiration, maintenance respiration, and relative growth rate. A case study with lettuce. Plant Cell Environ. 2003, 26, 1441–1449. [Google Scholar] [CrossRef]
- McCree, K.J. Equations for the Rate of Dark Respiration of White Clover and Grain Sorghum, as Functions of Dry Weight, Photosynthetic Rate, and Temperature1. Crop Sci. 1974, 14, 509–514. [Google Scholar] [CrossRef]
- Sarlikioti, V.; De Visser, P.H.; Marcelis, L.F. Exploring the spatial distribution of light interception and photosynthesis of canopies by means of a functional-structural plant model. Ann. Bot 2011, 107, 875–883. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, Ü. Photosynthesis and resource distribution through plant canopies. Plant Cell Environ. 2007, 30, 1052–1071. [Google Scholar] [CrossRef]
- Hikosaka, K. Leaf Canopy as a Dynamic System: Ecophysiology and Optimality in Leaf Turnover. Ann. Bot. 2004, 95, 521–533. [Google Scholar] [CrossRef] [Green Version]
- Boldt, J.K.; Meyer, M.H.; Erwin, J.E. Foliar Anthocyanins: A Horticultural Review. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 42, pp. 209–252. [Google Scholar]
- Das, P.K.; Geul, B.; Choi, S.B.; Yoo, S.D.; Park, Y.I. Photosynthesis-dependent anthocyanin pigmentation in Arabidopsis. Plant Signal. Behav. 2011, 6, 23–25. [Google Scholar] [CrossRef] [Green Version]
- Nichelmann, L.; Bilger, W. Quantification of light screening by anthocyanins in leaves of Berberis thunbergii. Planta 2017, 246, 1069–1082. [Google Scholar] [CrossRef]
- Tattini, M.; Sebastiani, F.; Brunetti, C.; Fini, A.; Torre, S.; Gori, A.; Centritto, M.; Ferrini, F.; Landi, M.; Guidi, L. Dissecting molecular and physiological response mechanisms to high solar radiation in cyanic and acyanic leaves: A case study on red and green basil. J. Exp. Bot 2017, 68, 2425–2437. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.-M. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Kang, J.H.; KrishnaKumar, S.; Atulba, S.L.S.; Jeong, B.R.; Hwang, S.J. Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 501–509. [Google Scholar] [CrossRef]
- Kim, C.; Van Iersel, M.W. The Quantum Requirement for CO2 Assimilation Increases with Increasing Photosynthetic Photon Flux Density and Leaf Anthocyanin Concentration in Lettuce. In Proceedings of the 2020 ASHS Annual Conference, Orlando, FL, USA, 1 September 2020; p. S154. [Google Scholar]
- Nielsen, S.L.; Simonsen, A.M. Photosynthesis and photoinhibition in two differently coloured varieties of Oxalis triangularis—the effect of anthocyanin content. Photosynthetica 2011, 49, 346–352. [Google Scholar] [CrossRef]
- McClain, A.M.; Sharkey, T.D. Building a better equation for electron transport estimated from Chl fluorescence: Accounting for nonphotosynthetic light absorption. New Phytol. 2020, 225, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Van Iersel, M.W. Photochemical Acclimation of Three Contrasting Species to Different Light Levels: Implications for Optimizing Supplemental Lighting. J. Am. Soc. Hortic. Sci. 2017, 142, 346–354. [Google Scholar] [CrossRef] [Green Version]








| Growth Parameters | Mean ± Standard Deviation | t | p-Value | |
|---|---|---|---|---|
| Green Cultivars | Red Cultivars | |||
| Dry weight (g) | 10.8 ± 3.4 | 7.9 ± 1.5 | 5.4 | <0.001 |
| Total leaf area (cm2) | 3223 ± 853 | 3393 ± 806 | −1.0 | 0.300 |
| Specific leaf area (cm2 g−1) | 307.2 ± 68.6 | 426.3 ± 65.2 | −9.1 | <0.001 |
| Canopy overlap ratio (m2 m−2) | 5.6 ± 2.5 | 5.6 ± 1.3 | −0.1 | 0.922 |
| Canopy ETR (mol) | 2.5 ± 0.8 | 3.3 ± 0.5 | −5.8 | <0.001 |
| Total incidental light (mol) | 18.6 ± 7.7 | 17.3 ± 2.6 | 1.1 | 0.287 |
| Light use efficiency (g mol−1) | 0.61 ± 0.10 | 0.46 ± 0.06 | 9.3 | <0.001 |
| Calculated ETR at PPFD of 200 (µmol m−2 s−1) | 48.9 ± 2.7 | 53.9 ± 2.4 | −9.9 | <0.001 |
| Calculated ETR at PPFD of 1000 (µmol m−2 s−1) | 110.9 ± 10.6 | 212.9 ± 68.5 | −11.3 | <0.001 |
| Projected canopy size at 13 DAG (cm2) | 4.8 ± 1.7 | 4.1 ± 1.1 | 2.1 | 0.037 |
| DAG | Statistical Summary | Regression Equation | ||||||
|---|---|---|---|---|---|---|---|---|
| Green 1 | Red 2 | Green | Red | |||||
| R2 | p-Value | R2 | p-Value | Intercept (g) | Slope (g cm−2) | Intercept (g) | Slope (g cm−2) | |
| 6 | 0.20 | 0.001 | 0.10 | 0.015 | 5.1 | 5.444 | 9.4 | −1.815 |
| 10 | 0.15 | 0.006 | 0.07 | 0.046 | 7.2 | 1.346 | 9.1 | −0.495 |
| 13 | 0.74 | <0.001 | 0.00 | 0.794 | 2.7 | 1.697 | ns 3 | ns |
| 17 | 0.20 | <0.001 | 0.02 | 0.292 | 6.2 | 0.346 | ns | ns |
| 20 | 0.38 | <0.001 | 0.00 | 0.800 | 4.5 | 0.246 | ns | ns |
| 24 | 0.45 | <0.001 | 0.00 | 0.712 | 4.3 | 0.126 | ns | ns |
| 28 | 0.50 | <0.001 | 0.01 | 0.414 | 4.3 | 0.084 | ns | ns |
| 32 | 0.67 | <0.001 | 0.22 | <0.001 | 4.0 | 0.052 | 4.0 | 0.031 |
| 34 | 0.74 | <0.001 | 0.31 | <0.001 | 3.8 | 0.041 | 3.7 | 0.026 |
| 38 | 0.89 | <0.001 | 0.53 | <0.001 | 3.8 | 0.024 | 2.6 | 0.019 |
| 41 | 0.91 | <0.001 | 0.51 | <0.001 | 3.6 | 0.017 | 1.9 | 0.016 |
| 44 | 0.87 | <0.001 | 0.52 | <0.001 | 3.8 | 0.013 | 1.0 | 0.014 |
| 48 | 0.76 | <0.001 | 0.45 | <0.001 | 4.3 | 0.010 | 1.7 | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; van Iersel, M.W. Morphological and Physiological Screening to Predict Lettuce Biomass Production in Controlled Environment Agriculture. Remote Sens. 2022, 14, 316. https://doi.org/10.3390/rs14020316
Kim C, van Iersel MW. Morphological and Physiological Screening to Predict Lettuce Biomass Production in Controlled Environment Agriculture. Remote Sensing. 2022; 14(2):316. https://doi.org/10.3390/rs14020316
Chicago/Turabian StyleKim, Changhyeon, and Marc W. van Iersel. 2022. "Morphological and Physiological Screening to Predict Lettuce Biomass Production in Controlled Environment Agriculture" Remote Sensing 14, no. 2: 316. https://doi.org/10.3390/rs14020316