Spectral Reflectance-Based Mangrove Species Mapping from WorldView-2 Imagery of Karimunjawa and Kemujan Island, Central Java Province, Indonesia
Abstract
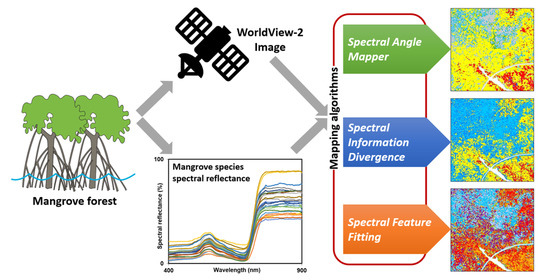
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Image Data and Processing
2.3. Field Data Collection
2.4. Mangrove Species Clustering Analysis
2.5. Pixel-Based Classification and Accuracy Assessment
3. Results
3.1. Spectral Reflectance of Mangrove Species
3.2. Clustering Analysis of Mangrove Species
3.3. Pixel-Based Classification
4. Discussion
4.1. Mangrove Species Clusters
4.2. Accuracy Assessment of the Resulting Maps
4.3. Classification Performance Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunting, P.; Rosenqvist, A.; Lucas, R.M.; Rebelo, L.-M.; Hilarides, L.; Thomas, N.; Hardy, A.; Itoh, T.; Shimada, M.; Finlayson, C.M. The Global Mangrove Watch—A New 2010 Global Baseline of Mangrove Extent. Remote Sens. 2018, 10, 1669. [Google Scholar] [CrossRef] [Green Version]
- Kusmana, C. Distribution and Current Status of Mangrove Forests in Indonesia. In Mangrove Ecosystems of Asia: Status, Challenges and Management Strategies; Faridah-Hanum, I., Latiff, A., Hakeem, K.R., Ozturk, M., Eds.; Springer: New York, NY, USA, 2014; pp. 37–60. [Google Scholar] [CrossRef]
- Spalding, M.; Kainuma, M.; Collins, L. World Atlas of Mangroves; Earthscan: London, UK, 2010. [Google Scholar]
- Karimunjawa National Park. Jenis Mangrove Taman Nasional Karimunjawa [Mangrove Species in the Karimunjawa National Park]; Karimunjawa National Park: Semarang, Indonesia, 2012. (In Indonesian) [Google Scholar]
- Ilman, M.; Dargusch, P.; Dart, P. A historical analysis of the drivers of loss and degradation of Indonesia’s mangroves. Land Use Policy 2016, 54, 448–459. [Google Scholar] [CrossRef]
- Lewis, D.; Phinn, S.; Arroyo, L. Cost-effectiveness of seven approaches to map vegetation communities—A case study from Northern Australia’s tropical savannas. Remote Sens. 2013, 5, 377–414. [Google Scholar] [CrossRef] [Green Version]
- Kuenzer, C.; Bluemel, A.; Gebhardt, S.; Quoc, T.V.; Dech, S. Remote sensing of mangrove ecosystems: A review. Remote Sens. 2011, 3, 878–928. [Google Scholar] [CrossRef] [Green Version]
- Lucas, R.; Lule, A.V.; Rodríguez, M.T.; Kamal, M.; Thomas, N.; Asbridge, E.; Kuenzer, C. Spatial Ecology of Mangrove Forests: A Remote Sensing Perspective. In Mangrove Ecosystems: A Global Biogeographic Perspective: Structure, Function, and Services; Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Twilley, R.R., Eds.; Springer: Cham, Switzerland, 2017; pp. 87–112. [Google Scholar]
- Vaiphasa, C.; Ongsomwang, S.; Vaiphasa, T.; Skidmore, A.K. Tropical mangrove species discrimination using hyperspectral data: A laboratory study. Estuar. Coast Shelf Sci. 2005, 65, 371–379. [Google Scholar] [CrossRef]
- Alwidakdo, A.; Azham, Z.; Kamarubayana, L. Studi Pertumbuhan Mangrove Pada Kegiatan Rehabilitasi Hutan Mangrove. Agrifor Jurnal Ilmu Pertanian dan Kehutanan 2014, 13, 11–18. (In Indonesian) [Google Scholar]
- Department of Forestry. Buku Informasi 50 Taman Nasional di Indonesia. In Book of Information on 50 National Parks in Indonesia; Directorate General of Forest Protection and Nature Conservation: Jakarta, Indonesia, 2007. (In Indonesian) [Google Scholar]
- Musa, M.; Handoyo, G.; Setyono, H. Peramalan Pasang di Perairan Pulau Karimunjawa, Kabupaten Jepara, Menggunakan Program “Worldtides”. J. Oceanogr. 2013, 3, 1–7. (In Indonesian) [Google Scholar]
- Data Sheet WorldView-2. Available online: https://dg-cms-uploads-production.s3.amazonaws.com/uploads/document/file/98/WorldView2-DS-WV2-rev2.pdf (accessed on 1 October 2016).
- Updike, T.; Comp, C. Radiometric Use of WorldView-2 Imagery. Available online: https://www.digitalglobe.com/sites/default/files/Radiometric_Use_of_WorldView-2_Imagery%20%281%29.pdf (accessed on 1 August 2013).
- Matthew, M.W.; Adler-Golden, S.M.; Berk, A.; Richtsmeier, S.C.; Levine, R.Y.; Bernstein, L.S.; Acharya, P.K.; Anderson, G.P.; Felde, G.W.; Hoke, M.P.; et al. Status of Atmospheric Correction Using a MODTRAN4-Based Algorithm. In Proceedings of the SPIE 4049 Algorithms for Multispectral, Hyperspectral, and Ultraspectral Imagery VI, Orlando, FL, USA, 23 August 2000. [Google Scholar]
- Level 1 and Atmosphere Archive and Distribution System (LAADS) DAAC. Available online: https://earthdata.nasa.gov/eosdis/daacs/laads (accessed on 15 December 2020).
- Boardman, J.W. Automated Spectral Unmixing of AVIRIS Data using Convex Geometry Concepts. In Proceedings of the Summaries of the 4th Annual JPL Air-Borne Geosciences Workshop, Pasadena, CA, USA, 25 October 1993; pp. 11–14. [Google Scholar]
- Plaza, A.J.; Chang, C.I. High Performance Computing in Remote Sensing; Chapman and Hall/CRC: Boca Raton, FL, USA, 2008. [Google Scholar]
- Kamal, M.; Arjasakusuma, S.; Adi, N.S. JAZ EL-350 VIS NIR Portable Spectrometer Operational Guideline; Remote Sensing Laboratory, Faculty of Geography Universitas Gadjah Mada: Yogyakarta, Indonesia, 2012. [Google Scholar]
- Kamal, M.; Ningam, M.U.L.; Alqorina, F. The Effect of Field Spectral Reflectance Measurement Distance to the Spectral Reflectance of Rhizophora stylosa. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Yogyakarta, Indonesia, 27–28 September 2017; Volume 98. [Google Scholar]
- Kamal, M.; Ningam, M.U.L.; Alqorina, F.; Wicaksono, P.; Murti, S.H. Combining field and image spectral reflectance for mangrove species identification and mapping using WorldView-2 image. In Proceedings of the SPIE Remote Sensing 10790, Earth Resources and Environmental Remote Sensing/GIS Applications IX, 107901P, Berlin, Germany, 9 October 2018. [Google Scholar]
- Wicaksono, P.; Fauzan, M.A.; Kumara, I.S.W.; Yogyantoro, R.N.; Lazuardi, W.; Zhafarina, Z. Analysis of reflectance spectra of tropical seagrass species and their value for mapping using multispectral satellite images. Int. J. Remote Sens. 2019, 40, 8955–8978. [Google Scholar] [CrossRef]
- Kruse, F.A.; Lefkoff, A.B.; Boardman, J.W.; Heidebrecht, K.B.; Shapiro, A.T.; Barloon, P.J.; Goetz, A.F.H. The Spectral Image Processing System (SIPS)—Interactive Visualization and Analysis of Imaging Spectrometer Data. Remote Sens. Environ. 1993, 44, 145–163. [Google Scholar] [CrossRef]
- Borengasser, M.; Hungate, W.S.; Watkins, R. Hyperspectral Remote Sensing: Principles and Applications; Taylor & Francis in Remote Sensing Applications, CRC Press: New York, NY, USA, 2008. [Google Scholar]
- Chang, C.I. An Information-Theoretic Approach to Spectral Variability, Similarity, and Discrimination for Hyperspectral Image Analysis. IEEE Tras. Inf. Theory 2000, 46, 1927–1932. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.N.; Swayze, G.A.; Gallagher, A.; Gorelick, N.; Kruse, F.A. Mapping with imaging spectrometer data using the complete band shape least-squares algorithm simultaneously fit to multiple spectral features from multiple materials. In Proceedings of the 3rd Airborne Visible/Infrared Imaging Spectrometer (AVIRIS) Workshop; JPL Publication 91-28; Jet Propulsion Laboratory: Pasadena, CA, USA, 20–21 May 1991; pp. 2–3. [Google Scholar]
- Congalton, R.G.; Green, K. Assessing the Accuracy of Remotely Sensed Data: Principles and Practices, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Klančnik, K.; Gaberščik, A. Leaf spectral signatures differ in plant species colonizing habitats along a hydrological gradient. J. Plant Ecol. 2016, 9, 442–450. [Google Scholar] [CrossRef]
- Andréfouët, S.; Kramer, P.; Torres-Pulliza, D.; Joyce, K.E.; Hochberg, E.J.; Garza-Pérez, R.; Muller-Karger, F.E. Multi-site evaluation of IKONOS data for classification of tropical coral reef environments. Remote Sens. Environ. 2003, 88, 128–143. [Google Scholar] [CrossRef]
- Kamal, M.; Phinn, S.; Johansen, K. Object-Based Approach for Multi-Scale Mangrove Composition Mapping Using Multi-Resolution Image Datasets. Remote Sens. 2015, 7, 4753–4783. [Google Scholar] [CrossRef] [Green Version]
- Hirano, A.; Madden, M.; Welch, R. Hyperspectral image data for mapping wetland vegetation. Wetland 2003, 23, 436–448. [Google Scholar] [CrossRef]
- Rashmi, S.; Addamani, S.; Venkat, S. Spectral Angle Mapper Algorithm for Seagrass and Other Benthic Habitats in Bolinao, Pangasinan using Worldview-2 Satellite Image. In Proceedings of the Geoscience and Remote Sensing Symposium (IGARSS): IEEE International, Melbourne, Australia, 21–26 July 2013; pp. 1579–1582. [Google Scholar] [CrossRef]
- Rahmandhana, A.D. Pemetaan Distribusi Jenis Mangrove Melalui Integrasi Citra WorldView-2 dan Pengukuran Spektrometer Lapangan di Pulau Karimunjawa dan Kemujan, Kabupaten Jepara [Mapping the Distribution of Mangrove Species through the Integration of WorldView-2 Image and Field Spectrometer Measurements in Karimunjawa and Kemujan Island, Jepara Regency]. Master’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 14 September 2021. (In Indonesian). [Google Scholar]
- Muhammad, S.; Mirza, M.W. Hyperspectral Mapping Methods for Differentiating Mangrove Species along Karachi Coast. Int. J. Environ. Ecol. Eng. 2013, 7, 963–965. [Google Scholar] [CrossRef]
- Nidamanuri, R.R.; Zbell, B. A method for selecting optimal spectral resolution and comparison metric for material mapping by spectral library search. Prog. Phys. Geog. 2010, 34, 47–58. [Google Scholar] [CrossRef]
- Shanmugam, S.; Srinivasaperumal, P. Spectral Matching Approaches in Hyperspectral Image Processing. Int. J. Remote Sens. 2014, 35, 8217–8251. [Google Scholar] [CrossRef]
- Hyperspectral Analytics in ENVI Target Detection and Spectral Mapping Methods. Available online: http://www.spectroexpo.com/wp-content/uploads/2021/03/Hyperspectral_Whitepaper.pdf (accessed on 10 December 2020).






| Spectral Band | Wavelength | Spatial Resolution |
|---|---|---|
| Panchromatic | 450–800 nm | 0.46 m |
| Multispectral–Coastal | 400–450 nm | 1.84 m |
| Multispectral–Blue | 450–510 nm | 1.84 m |
| Multispectral–Green | 510–580 nm | 1.84 m |
| Multispectral–Yellow | 585–625 nm | 1.84 m |
| Multispectral–Red | 630–690 nm | 1.84 m |
| Multispectral–Red Edge | 705–745 nm | 1.84 m |
| Multispectral–Near-IR1 | 770–895 nm | 1.84 m |
| Multispectral–Near-IR2 | 860–1040 nm | 1.84 m |
| No | Mangrove Species | No | Mangrove Species | No | Mangrove Species |
|---|---|---|---|---|---|
| 1 | Acanthus ebracteatus | 9 | Ceriops tagal | 17 | Rhizophora mucronata |
| 2 | Acanthus ilicifolius | 10 | Excoecaria agallocha | 18 | Rhizophora stylosa |
| 3 | Acrostichum aureum | 11 | Heritiera littoralis | 19 | Scyphiphora hydrophyllacea |
| 4 | Aegiceras corniculatum | 12 | Lumnitzera littorea | 20 | Sonneratia alba |
| 5 | Avicennia marina | 13 | Lumnitzera racemosa | 21 | Sonneratia caseolaris |
| 6 | Bruguiera cylindrica | 14 | Pemphis acidula | 22 | Sonneratia ovata |
| 7 | Bruguiera gymnorrhiza | 15 | Rhizophora apiculata | 23 | Xylocarpus granatum |
| 8 | Bruguiera sexangula | 16 | Rhizophora lamarckii | 24 | Xylocarpus moluccensis |
| Level | Group | Mangrove Species |
|---|---|---|
| Level 1 | A | A. ebracteatus, A. ilicifolius, A. aureum, A. corniculatum, A. marina, B. cylindrica, B. sexangular, C. tagal, H. littoralis, L. racemosa, P. acidula, R. mucronata, R. stylosa, S. hydrophyllacea, S. alba, X. moluccensis |
| B | B. gymnorrhiza, E. agallocha, L. littorea, R. apiculata, R. lamarckii, S. caseolaris, S. ovata, X. granatum | |
| Level 2 | A | A. ebracteatus, A. aureum, A. corniculatum, B. cylindrica, B. sexangular, C. tagal, H. littoralis, R. mucronata, R. stylosa, S. hydrophyllacea |
| B | A. ilicifolius, A. marina, L. racemosa, P. acidula, S. alba, X. moluccensis | |
| C | R. lamarckii, S. ovata | |
| D | B. gymnorrhiza, E. agallocha, L. littorea, R. apiculata, S. caseolaris, X. granatum | |
| Level 3 | A | A. corniculatum, B. cylindrica, R. mucronata, R. stylosa |
| B | A. ebracteatus, A. aureum, B. sexangula, C. tagal, H. littoralis, S. hydrophyllacea | |
| C | A. ilicifolius, A. marina, L. racemosa, P. acidula, S. alba, X. moluccensis | |
| D | R. lamarckii, S. ovata | |
| E | B. gymnorrhiza, E. agallocha, L. littorea, R. apiculata, S. caseolaris, X. granatum | |
| Level 4 | Individual mangrove species |
| Classification Algorithms | Level 1 (%) | Level 2 (%) | Level 3 (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | PA | UA | OA | Group | PA | UA | OA | Group | PA | UA | OA | |
| SAM | A | 8.40 | 47.62 | 7.34 | A | 5.88 | 50.00 | 5.08 | A | 3.33 | 25.00 | 5.65 |
| B | 5.17 | 37.50 | B | 5.88 | 15.00 | B | 13.16 | 33.33 | ||||
| C | 0.00 | 0.00 | C | 5.88 | 21.43 | |||||||
| D | 3.85 | 16.67 | D | 0.00 | 0.00 | |||||||
| E | 1.92 | 8.33 | ||||||||||
| SID | A | 58.82 | 66.67 | 49.72 | A | 36.76 | 31.25 | 22.60 | A | 13.33 | 5.97 | 15.25 |
| B | 31.03 | 39.13 | B | 3.92 | 7.14 | B | 42.11 | 27.59 | ||||
| C | 0.00 | 0.00 | C | 1.96 | 5.26 | |||||||
| D | 25.00 | 30.23 | D | 0.00 | 0.00 | |||||||
| E | 11.54 | 35.29 | ||||||||||
| SFF | A | 21.85 | 70.27 | 38.42 | A | 10.29 | 31.82 | 10.73 | A | 6.67 | 4.65 | 7.91 |
| B | 72.41 | 43.30 | B | 5.88 | 7.50 | B | 5.26 | 50.00 | ||||
| C | 50.00 | 0.00 | C | 5.88 | 7.89 | |||||||
| D | 11.54 | 20.69 | D | 50.00 | 6.25 | |||||||
| E | 7.69 | 23.53 | ||||||||||
| Class | SAM | SID | SFF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PA (%) | UA (%) | OA (%) | PA (%) | UA (%) | OA (%) | PA (%) | UA (%) | OA (%) | |
| A | 0 | 0 | 0.56 | 0 | 0 | 0 | 0 | 0 | 5.08 |
| B | 0 | 0 | 0 | 0 | 0 | 0 | |||
| C | 0 | 0 | 0 | 0 | 0 | 0 | |||
| D | 0 | 0 | 0 | 0 | 0 | 0 | |||
| E | 0 | 0 | 0 | 0 | 0 | 0 | |||
| F | 0 | 0 | 0 | 0 | 0 | 0 | |||
| G | 0 | 0 | 0 | 0 | 0 | 0 | |||
| H | 0 | 0 | 0 | 0 | 33.33 | 2.27 | |||
| I | 0 | 0 | 0 | 0 | 0 | 0 | |||
| J | 0 | 0 | 0 | 0 | 6.25 | 9.09 | |||
| K | 0 | 0 | 0 | 0 | 0 | 0 | |||
| L | 0 | 0 | 0 | 0 | 46.15 | 37.50 | |||
| M | 0 | 0 | 0 | 0 | 0 | 0 | |||
| N | 0 | 0 | 0 | 0 | 0 | 0 | |||
| O | 0 | 0 | 0 | 0 | 0 | 0 | |||
| P | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Q | 0 | 0 | 0 | 0 | 0 | 0 | |||
| R | 0 | 0 | 0 | 0 | 0 | 0 | |||
| S | 0 | 0 | 0 | 0 | 0 | 0 | |||
| T | 0 | 0 | 0 | 0 | 0 | 0 | |||
| U | 33.33 | 2.44 | 0 | 0 | 33.33 | 16.67 | |||
| V | 0 | 0 | 0 | 0 | 0 | 0 | |||
| W | 0 | 0 | 0 | 0 | 0 | 0 | |||
| X | 0 | 0 | 0 | 0 | 0 | 0 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmandhana, A.D.; Kamal, M.; Wicaksono, P. Spectral Reflectance-Based Mangrove Species Mapping from WorldView-2 Imagery of Karimunjawa and Kemujan Island, Central Java Province, Indonesia. Remote Sens. 2022, 14, 183. https://doi.org/10.3390/rs14010183
Rahmandhana AD, Kamal M, Wicaksono P. Spectral Reflectance-Based Mangrove Species Mapping from WorldView-2 Imagery of Karimunjawa and Kemujan Island, Central Java Province, Indonesia. Remote Sensing. 2022; 14(1):183. https://doi.org/10.3390/rs14010183
Chicago/Turabian StyleRahmandhana, Arie Dwika, Muhammad Kamal, and Pramaditya Wicaksono. 2022. "Spectral Reflectance-Based Mangrove Species Mapping from WorldView-2 Imagery of Karimunjawa and Kemujan Island, Central Java Province, Indonesia" Remote Sensing 14, no. 1: 183. https://doi.org/10.3390/rs14010183