Author Contributions
Conceptualization, G.R. and A.G.; methodology, G.R. and R.G.; software, M.V. and G.R.; validation, G.R., R.G. and A.G.; formal analysis, G.R. and R.G.; investigation, G.R., R.G. and M.V.; resources, M.S. and S.L.; writing—original draft preparation, G.R.; writing—review and editing, G.R., M.S., A.G. and S.L.; visualization, G.R. and R.G.; supervision, M.S. and S.L.; project administration, G.R.; funding acquisition, M.S. and S.L. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Concentration of ion species over decreasing pH value calculated using the Henderson–Hasselbach equation and pKa value of 10.14. The pH value was measured at the column outlet for the following experimental conditions: 25 °C, 300 rpm, 1 L minSTP−1 of 30 vol% CO2 and 70 vol% N2 gas mixture and 0.18 mL min−1 gas/liquid flow and 0.1 molar NaOH in the feed.
Figure 1.
Concentration of ion species over decreasing pH value calculated using the Henderson–Hasselbach equation and pKa value of 10.14. The pH value was measured at the column outlet for the following experimental conditions: 25 °C, 300 rpm, 1 L minSTP−1 of 30 vol% CO2 and 70 vol% N2 gas mixture and 0.18 mL min−1 gas/liquid flow and 0.1 molar NaOH in the feed.
Figure 2.
PID sketch of the experimental setup. The setup includes the holdup measurement with differential pressure transducer (PDT) and the oxygen probes (O21 and O22) for kLa determination. The CO2 sensor on top of the column and the pH probe at the bottom of the column were used to monitor the chemisorption process in neutralization experiments.
Figure 2.
PID sketch of the experimental setup. The setup includes the holdup measurement with differential pressure transducer (PDT) and the oxygen probes (O21 and O22) for kLa determination. The CO2 sensor on top of the column and the pH probe at the bottom of the column were used to monitor the chemisorption process in neutralization experiments.
Figure 3.
Left: Perforated rotor disc for gas/liquid contact. The rotor discs are made of stainless steel with a thickness of 1 mm. All dimensions are given in mm. Right: schematic drawing of a single compartment. Dimensions are summarized in
Table 1.
Figure 3.
Left: Perforated rotor disc for gas/liquid contact. The rotor discs are made of stainless steel with a thickness of 1 mm. All dimensions are given in mm. Right: schematic drawing of a single compartment. Dimensions are summarized in
Table 1.
Figure 4.
Molar ratio of CO2 vs. NaOH feed for constant pH of pH 9 at the column outlet.
Figure 4.
Molar ratio of CO2 vs. NaOH feed for constant pH of pH 9 at the column outlet.
Figure 5.
Measured pH value at the column outlet over time and corresponding CO2 concentration in ppm in the exhaust gas for a reaction temperature of 25 °C and gas feed of 1 L minSTP−1 (30 vol% CO2 and 70 vol% N2); stirrer speed 300 rpm and 60 mL min−1 0.1 molar NaOH feed rate until pH 9 was obtained. At pH 9, the NaOH feed was changed to 180 mL min−1.
Figure 5.
Measured pH value at the column outlet over time and corresponding CO2 concentration in ppm in the exhaust gas for a reaction temperature of 25 °C and gas feed of 1 L minSTP−1 (30 vol% CO2 and 70 vol% N2); stirrer speed 300 rpm and 60 mL min−1 0.1 molar NaOH feed rate until pH 9 was obtained. At pH 9, the NaOH feed was changed to 180 mL min−1.
Figure 6.
Measured pH value at column outlet over time for different rotational speeds with a 60 mL min−1 0.1 molar NaOH feed at 25 °C and 1 L minSTP−1 of gas mixture (30 vol% CO2 and 70 vol% N2).
Figure 6.
Measured pH value at column outlet over time for different rotational speeds with a 60 mL min−1 0.1 molar NaOH feed at 25 °C and 1 L minSTP−1 of gas mixture (30 vol% CO2 and 70 vol% N2).
Figure 7.
Measured pH at the column outlet over time for different temperatures with a 60 mL min−1 feed rate of 0.1 molar NaOH, 500 rpm and 1 L minSTP−1 (30 vol% CO2 and 70 vol% N2) gas flow rate.
Figure 7.
Measured pH at the column outlet over time for different temperatures with a 60 mL min−1 feed rate of 0.1 molar NaOH, 500 rpm and 1 L minSTP−1 (30 vol% CO2 and 70 vol% N2) gas flow rate.
Figure 8.
Measured pH at the column outlet over time for different gas flow rates (30 vol% CO2 and 70 vol% N2) and 60 mL min−1 0.1 molar NaOH feed, 700 rpm and 25 °C.
Figure 8.
Measured pH at the column outlet over time for different gas flow rates (30 vol% CO2 and 70 vol% N2) and 60 mL min−1 0.1 molar NaOH feed, 700 rpm and 25 °C.
Figure 9.
Influence of temperature on rpm-averaged pH at the column outlet at steady-state operation.
Figure 9.
Influence of temperature on rpm-averaged pH at the column outlet at steady-state operation.
Figure 10.
Rpm-averaged CO2 concentration at the column outlet for different temperatures and different dispersed gas flow rates. Error bars show the influence of rotational speed.
Figure 10.
Rpm-averaged CO2 concentration at the column outlet for different temperatures and different dispersed gas flow rates. Error bars show the influence of rotational speed.
Figure 11.
Changing flow regimes for different rotational speeds at a constant gas feed rate of 2 L min
STP−1 and liquid feed flow rate of 0.28 L min
−1; (
a) at 300 rpm (mainly external flow); (
b) mixed flow at 500 rpm; (
c) internal flow only at 700 rpm. Videos of flow regime (
a) S1, (
b) S2 and (
c) S3 are included in the
Supplementary Materials.
Figure 11.
Changing flow regimes for different rotational speeds at a constant gas feed rate of 2 L min
STP−1 and liquid feed flow rate of 0.28 L min
−1; (
a) at 300 rpm (mainly external flow); (
b) mixed flow at 500 rpm; (
c) internal flow only at 700 rpm. Videos of flow regime (
a) S1, (
b) S2 and (
c) S3 are included in the
Supplementary Materials.
Figure 12.
Dispersed gas phase holdup φg in the TCDC for a constant water flow rate of 245 mL min−1 and a different flow rate of synthetic air (1 L minSTP−1, 2 L minSTP−1, 3 L minSTP−1).
Figure 12.
Dispersed gas phase holdup φg in the TCDC for a constant water flow rate of 245 mL min−1 and a different flow rate of synthetic air (1 L minSTP−1, 2 L minSTP−1, 3 L minSTP−1).
Figure 13.
Dispersed gas phase holdup and rotational speed at different volumetric flow rates at 25 °C and aqueous phase feed according to
Table 6.
Figure 13.
Dispersed gas phase holdup and rotational speed at different volumetric flow rates at 25 °C and aqueous phase feed according to
Table 6.
Figure 14.
Normalized residence time distribution at 300 rpm, 1 L minSTP−1 air, and 180 mL min−1 water. The dots indicate the measured, normalized experimental data. The measured data were fitted with log-normal distribution (solid black line). The dotted line indicates the residence time distribution with 5 CSTRs in series, and the dashed line shows the modelled residence time distribution for 6 CSTRs in series.
Figure 14.
Normalized residence time distribution at 300 rpm, 1 L minSTP−1 air, and 180 mL min−1 water. The dots indicate the measured, normalized experimental data. The measured data were fitted with log-normal distribution (solid black line). The dotted line indicates the residence time distribution with 5 CSTRs in series, and the dashed line shows the modelled residence time distribution for 6 CSTRs in series.
Figure 15.
Influence of rotational speed at 25 °C on (
a) mean residence time t
exp compared with hydraulic residence time t
hydro; (
b) Bodenstein number; (
c) number of continuously stirred tanks in the cascade N
CSTR; (
d) axial dispersion coefficient D
ax. The corresponding aqueous phase flow rates for the varied gas flow rates are shown in
Table 6.
Figure 15.
Influence of rotational speed at 25 °C on (
a) mean residence time t
exp compared with hydraulic residence time t
hydro; (
b) Bodenstein number; (
c) number of continuously stirred tanks in the cascade N
CSTR; (
d) axial dispersion coefficient D
ax. The corresponding aqueous phase flow rates for the varied gas flow rates are shown in
Table 6.
Figure 16.
Influence of rotational speed on kLa at 25 °C under variation of gas and corresponding liquid flow rates of 0.18 mL min−1 for 1 L minSTP−1; 0.32 mL min−1 for 2 L minSTP−1 and 0.42 L min−1 for 3 L minSTP−1.
Figure 16.
Influence of rotational speed on kLa at 25 °C under variation of gas and corresponding liquid flow rates of 0.18 mL min−1 for 1 L minSTP−1; 0.32 mL min−1 for 2 L minSTP−1 and 0.42 L min−1 for 3 L minSTP−1.
Figure 17.
Influence of air flow rate Vg on kLa at a rotational speed of 500 rpm. Dotted lines indicate the linear trend of increasing kLa over gas flow rate.
Figure 17.
Influence of air flow rate Vg on kLa at a rotational speed of 500 rpm. Dotted lines indicate the linear trend of increasing kLa over gas flow rate.
Figure 18.
Temperature-dependent kLa at 500 rpm. The lines show the fitted temperature dependency with β = 0.019 (Equation (25)). The single markers indicate the experimental data points.
Figure 18.
Temperature-dependent kLa at 500 rpm. The lines show the fitted temperature dependency with β = 0.019 (Equation (25)). The single markers indicate the experimental data points.
Figure 19.
Parity plot of the measured CO2 consumption in the reactor vs. the modelled CO2 consumption using the fitted pKa values: 25 °C, pKa = 10.14; 40 °C, pKa = 10.29; 60 °C, pKa = 10.36.
Figure 19.
Parity plot of the measured CO2 consumption in the reactor vs. the modelled CO2 consumption using the fitted pKa values: 25 °C, pKa = 10.14; 40 °C, pKa = 10.29; 60 °C, pKa = 10.36.
Figure 20.
Overall CO2 conversion X in the reactor vs. Vc/nCO2 at 25 °C. The slope of the dashed lines gives the average reaction rate in the reactor: 1 L minSTP−1 = 0.13 mol m−3 s−1; 2 L minSTP−1 = 0.36 mol m−3 s−1; 3 L minSTP−1 = 0.56 mol m−3 s−1.
Figure 20.
Overall CO2 conversion X in the reactor vs. Vc/nCO2 at 25 °C. The slope of the dashed lines gives the average reaction rate in the reactor: 1 L minSTP−1 = 0.13 mol m−3 s−1; 2 L minSTP−1 = 0.36 mol m−3 s−1; 3 L minSTP−1 = 0.56 mol m−3 s−1.
Figure 21.
Influence of rotational speed and gas flow rate on the reaction rate.
Table 6 shows the corresponding aqueous phase feed to the gas flow rate (30 vol% CO
2 and 70 vol% N
2).
Figure 21.
Influence of rotational speed and gas flow rate on the reaction rate.
Table 6 shows the corresponding aqueous phase feed to the gas flow rate (30 vol% CO
2 and 70 vol% N
2).
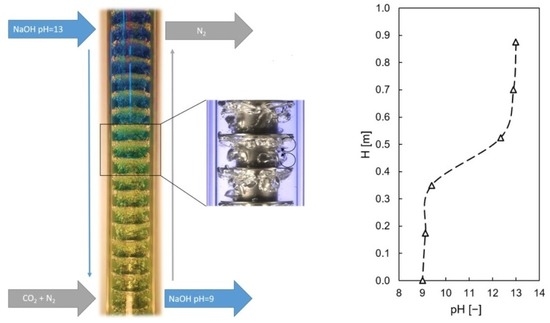
Figure 22.
Model assumption for continuous chemisorption of CO2 in 0.1 molar NaOH solution in the Taylor-Couette disc contactor. The neutralization process over reactor height was visualized by adding a liquid universal pH indicator. Blue indicates pH 14 at the column inlet and yellow indicates a steady-state pH of pH 9 at the column outlet.
Figure 22.
Model assumption for continuous chemisorption of CO2 in 0.1 molar NaOH solution in the Taylor-Couette disc contactor. The neutralization process over reactor height was visualized by adding a liquid universal pH indicator. Blue indicates pH 14 at the column inlet and yellow indicates a steady-state pH of pH 9 at the column outlet.
Figure 23.
Modelled CO2 molar flow rate (dashed line) over column height at steady-state operation for the experimental parameters of 25 °C, 500 rpm, 3 L min STP−1 gas feed (30 vol% CO2 and 70 vol% N2), 0.42 L min−1 of 0.1 molar NaOH feed with 5 measured CSTRs in a cascade. The dots indicate the molar CO2 flow rate obtained in the individual CSTRs according to the pH with a pKa of 10.14 and 5 CSTRS along the active column height.
Figure 23.
Modelled CO2 molar flow rate (dashed line) over column height at steady-state operation for the experimental parameters of 25 °C, 500 rpm, 3 L min STP−1 gas feed (30 vol% CO2 and 70 vol% N2), 0.42 L min−1 of 0.1 molar NaOH feed with 5 measured CSTRs in a cascade. The dots indicate the molar CO2 flow rate obtained in the individual CSTRs according to the pH with a pKa of 10.14 and 5 CSTRS along the active column height.
Figure 24.
(a) Modelled pH trajectory over column height for the neutralization experiment at 25 °C, 500 rpm and gas flow rate of 3 L minSTP−1 and corresponding enhancement factor E [-] calculated for each CSTR; (b) corresponding concentration of ion species with pKa 10.14. The markers indicate the concentration and pH in the individual CSTR.
Figure 24.
(a) Modelled pH trajectory over column height for the neutralization experiment at 25 °C, 500 rpm and gas flow rate of 3 L minSTP−1 and corresponding enhancement factor E [-] calculated for each CSTR; (b) corresponding concentration of ion species with pKa 10.14. The markers indicate the concentration and pH in the individual CSTR.
Table 1.
Geometric data of the lab-scale TCDC DN 50 column.
Table 1.
Geometric data of the lab-scale TCDC DN 50 column.
| TCDC DN50 |
|---|
| active length | H | 0.875 | [m] |
| column diameter | dC | 0.05 | [m] |
| shaft diameter | dSh | 0.025 | [m] |
| number of compartments | N | 24 | [-] |
| compartment height | HC | 0.025 | [m] |
| column volume | VC | 1.35 | [l] |
| rotor disc diameter | dR | 0.043 | [m] |
Table 2.
Coefficients of the power series to calculate O
2 solubility with Equation (18) as a function of temperature (0–50 °C) [
21].
Table 2.
Coefficients of the power series to calculate O
2 solubility with Equation (18) as a function of temperature (0–50 °C) [
21].
| Parameter | Value |
|---|
| a | 4.900·10−2 |
| b | −1.335·10−3 |
| c | 2.759·10−5 |
| d | 3.235·10−7 |
| e | 1.614·10−9 |
Table 3.
Ion specific parameters according to Weisenberger and Schumpe [
26].
Table 3.
Ion specific parameters according to Weisenberger and Schumpe [
26].
| Ion | hi [m3 kmol−1] | Gas | hg [m3 kmol−1] | bi [m3 kmol−1] |
|---|
| Na+ | 0.1143 | CO2 | −0.0172 | −0.0857 |
| OH− | 0.0839 | | | −0.1088 |
| 0.0967 | | | −0.115 |
| 0.1423 | | | −0.245 |
Table 4.
Parameters for determination of OH
− diffusivity [
24].
Table 4.
Parameters for determination of OH
− diffusivity [
24].
| ψ | | T0 [K] |
|---|
| 1.658 | 2.665·10−8 | 216.5 |
Table 5.
Chemicals used for neutralization experiments and hydraulic characterization.
Table 5.
Chemicals used for neutralization experiments and hydraulic characterization.
| Name | Manufacturer | CAS | Purity |
|---|
| sodium hydroxide | J.T. Baker | 1310-73-2 | ≥98% |
| deionized water | in-house source | | |
| synthetic air | Air liquide | | ≥99% |
| nitrogen | Air liquide | 7727-37-9 | ≥99.8% |
| gas mix 30% CO2 and 70% N2 | Air liquide | | ≥99% |
Table 6.
List of neutralization experiments.
Table 6.
List of neutralization experiments.
| | | |
|---|
| [°C] | [L min−1] | [L minSTP−1] | [L min−1] |
|---|
| 25 | 300 | 1 | 0.18 |
| 400 |
| 700 |
| 25 | 300 | 2 | 0.28 |
| 400 |
| 700 |
| 25 | 300 | 3 | 0.42 |
| 400 |
| 700 |
| 40 | 300 | 1 | 0.18 |
| 400 |
| 700 |
| 40 | 300 | 2 | 0.28 |
| 400 |
| 700 |
| 40 | 300 | 3 | 0.42 |
| 400 |
| 700 |
| 60 | 300 | 1 | 0.18 |
| 400 |
| 700 |
| 60 | 300 | 2 | 0.28 |
| 400 |
| 700 |
| 60 | 300 | 3 | 0.42 |
| 400 |
| 700 |