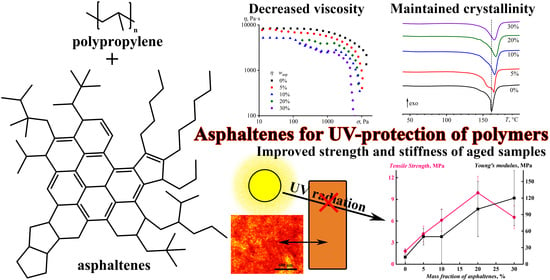
Asphaltenes from Heavy Crude Oil as Ultraviolet Stabilizers against Polypropylene Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Structure of Asphaltene/Polypropylene Composites
3.2. Rheological Properties of Asphaltene/Polypropylene Composites
3.3. Calorimetry of Asphaltene/Polypropylene Composites
3.4. Strength Properties of Asphaltene/Polypropylene Composites
4. Conclusions
- Asphaltenes act as plasticizers for polypropylene, slightly reducing its viscosity, melting point, crystallinity, tensile strength, and stiffness, most likely due to their limited solubility in the polymer medium and low molecular weight;
- UV exposure dramatically reduces the viscosity and strength of polypropylene because of numerous breaks in the polymer chains, although their crystallinity is maintained, making polypropylene films brittle due to a drop in molecular weight and its approach to the molecular weight between entanglements;
- UV radiation and asphaltenes lead to the predominant formation of monoclinic crystals (α phase) and a decrease in the content of trigonal ones (β), which may be due to the break of chains and the action of asphaltenes as crystallization nucleators;
- Asphaltenes effectively absorb UV radiation, suppressing the destruction of polymer chains several times and vastly increasing the strength and rigidity of aged films compared to asphaltene-free polypropylene after UV exposure;
- Although 30% of asphaltenes reduce the destruction of the polymer by seven times, they also decrease the strength of the polymer by half, possibly due to aggregation. Therefore, the optimal mass fraction of asphaltenes is 20%, which reduces the destruction of macromolecular chains by six times and gives the best strength of the polymer films after UV aging.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, R.; Li, K.; Lin, Y.; Lu, C.; Duan, X. Characterization Techniques of Polymer Aging: From Beginning to End. Chem. Rev. 2023, 123, 3007–3088. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L.; Hamid, H.S.; Torikai, A. Effects of Climate Change and UV-B on Materials. Photochem. Photobiol. Sci. 2003, 2, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Wypych, G. Handbook of UV Degradation and Stabilization, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2020; ISBN 978-1-927885-58-1. [Google Scholar] [CrossRef]
- Naebe, M.; Abolhasani, M.M.; Khayyam, H.; Amini, A.; Fox, B. Crack Damage in Polymers and Composites: A Review. Polym. Rev. 2016, 56, 31–69. [Google Scholar] [CrossRef]
- Lu, T.; Solis-Ramos, E.; Yi, Y.; Kumosa, M. UV Degradation Model for Polymers and Polymer Matrix Composites. Polym. Degrad. Stab. 2018, 154, 203–210. [Google Scholar] [CrossRef]
- Wypych, G. (Ed.) Handbook of UV Degradation and Stabilization; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-1-927885-57-4. [Google Scholar]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef]
- Yousif, E.; Ahmed, D.; Zainulabdeen, K.; Jawad, A. Photo-physical and Morphological Study of Polymers: A Review. Phys. Chem. Res. 2023, 11, 409–424. [Google Scholar] [CrossRef]
- Morabito, K.; Shapley, N.C.; Steeley, K.G.; Tripathi, A. Review of Sunscreen and the Emergence of Non-Conventional Absorbers and Their Applications in Ultraviolet Protection: Emergence of Non-Conventional Absorbers. Int. J. Cosmet. Sci. 2011, 33, 385–390. [Google Scholar] [CrossRef]
- Celina, M.C. Review of Polymer Oxidation and Its Relationship with Materials Performance and Lifetime Prediction. Polym. Degrad. Stab. 2013, 98, 2419–2429. [Google Scholar] [CrossRef]
- Bracco, P.; Costa, L.; Luda, M.P.; Billingham, N. A Review of Experimental Studies of the Role of Free-Radicals in Polyethylene Oxidation. Polym. Degrad. Stab. 2018, 155, 67–83. [Google Scholar] [CrossRef]
- Zhao, W.; He, J.; Yu, P.; Jiang, X.; Zhang, L. Recent Progress in the Rubber Antioxidants: A Review. Polym. Degrad. Stab. 2023, 207, 110223. [Google Scholar] [CrossRef]
- Pospíšil, J.; Klemchuk, P.P. (Eds.) Oxidation Inhibition in Organic Materials; CRC Press: Boca Raton, FL, USA, 1989; ISBN 978-0-8493-4767-2. [Google Scholar]
- Grum, J. Book Review: Plastics Additives Handbook, 5th Edition by H. Zweifel. IJMMP 2008, 3, 451. [Google Scholar] [CrossRef]
- Morimoto, T.; Tomonaga, H.; Mitani, A. Ultraviolet Ray Absorbing Coatings on Glass for Automobiles. Thin Solid. Film. 1999, 351, 61–65. [Google Scholar] [CrossRef]
- Jaroenworaluck, A.; Sunsaneeyametha, W.; Kosachan, N.; Stevens, R. Characteristics of Silica-Coated TiO2 and Its UV Absorption for Sunscreen Cosmetic Applications. Surf. Interface Anal. 2006, 38, 473–477. [Google Scholar] [CrossRef]
- Sakamoto, H.; Qiu, J.; Makishima, A. The Preparation and Properties of CeO2–TiO2 Film by Sol–Gel Spin-Coating Process. Sci. Technol. Adv. Mater. 2003, 4, 69–76. [Google Scholar] [CrossRef]
- Kundu, D.; Mukherjee, R. UV Absorbing Transparent Sol-Gel Derived Coatings on Glass. J. Mater. Sci. Lett. 2003, 22, 1647–1649. [Google Scholar] [CrossRef]
- Mahltig, B.; Böttcher, H.; Rauch, K.; Dieckmann, U.; Nitsche, R.; Fritz, T. Optimized UV Protecting Coatings by Combination of Organic and Inorganic UV Absorbers. Thin Solid. Film. 2005, 485, 108–114. [Google Scholar] [CrossRef]
- Weichelt, F.; Emmler, R.; Flyunt, R.; Beyer, E.; Buchmeiser, M.R.; Beyer, M. ZnO-Based UV Nanocomposites for Wood Coatings in Outdoor Applications: ZnO-Based UV Nanocomposites for Wood Coatings in outdoor applications. Macromol. Mater. Eng. 2010, 295, 130–136. [Google Scholar] [CrossRef]
- Gugumus, F. Possibilities and Limits of Synergism with Light Stabilizers in Polyolefins 2. UV Absorbers in Polyolefins. Polym. Degrad. Stab. 2002, 75, 309–320. [Google Scholar] [CrossRef]
- Maliakal, A.; Lem, G.; Turro, N.J.; Ravichandran, R.; Suhadolnik, J.C.; DeBellis, A.D.; Wood, M.G.; Lau, J. Twisted Intramolecular Charge Transfer States in 2-Arylbenzotriazoles: Fluorescence Deactivation via Intramolecular Electron Transfer Rather than Proton Transfer. J. Phys. Chem. A 2002, 106, 7680–7689. [Google Scholar] [CrossRef]
- McGarry, P.F.; Jockusch, S.; Fujiwara, Y.; Kaprinidis, N.A.; Turro, N.J. DMSO Solvent Induced Photochemistry in Highly Photostable Compounds. The Role of Intermolecular Hydrogen Bonding. J. Phys. Chem. A 1997, 101, 764–767. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light Blocker—A Review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, J.; Nešpurek, S. Photostabilization of Coatings. Mechanisms and Performance. Prog. Polym. Sci. 2000, 25, 1261–1335. [Google Scholar] [CrossRef]
- Hamid, S.H. Handbook of Polymer Degradation, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-0-429-08258-0. [Google Scholar]
- Väisänen, T.; Haapala, A.; Lappalainen, R.; Tomppo, L. Utilization of Agricultural and Forest Industry Waste and Residues in Natural Fiber-Polymer Composites: A Review. Waste Manag. 2016, 54, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Lacerda, T.M. From Monomers to Polymers from Renewable Resources: Recent Advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Maraveas, C. Production of Sustainable and Biodegradable Polymers from Agricultural Waste. Polymers 2020, 12, 1127. [Google Scholar] [CrossRef]
- Kamkar, M.; Natale, G. A Review on Novel Applications of Asphaltenes: A Valuable Waste. Fuel 2021, 285, 119272. [Google Scholar] [CrossRef]
- Mullins, O.C. The Asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 393–418. [Google Scholar] [CrossRef]
- Ilyin, S.; Arinina, M.; Polyakova, M.; Bondarenko, G.; Konstantinov, I.; Kulichikhin, V.; Malkin, A. Asphaltenes in Heavy Crude Oil: Designation, Precipitation, Solutions, and Effects on Viscosity. J. Pet. Sci. Eng. 2016, 147, 211–217. [Google Scholar] [CrossRef]
- Martínez-Palou, R.; Mosqueira, M.D.L.; Zapata-Rendón, B.; Mar-Juárez, E.; Bernal-Huicochea, C.; De La Cruz Clavel-López, J.; Aburto, J. Transportation of Heavy and Extra-Heavy Crude Oil by Pipeline: A Review. J. Pet. Sci. Eng. 2011, 75, 274–282. [Google Scholar] [CrossRef]
- Moud, A.A. Asphaltene Induced Changes in Rheological Properties: A Review. Fuel 2022, 316, 123372. [Google Scholar] [CrossRef]
- Demirbas, A. Deasphalting of Crude Oils Using Supercritical Fluids. Pet. Sci. Technol. 2016, 34, 665–670. [Google Scholar] [CrossRef]
- Magomedov, R.N.; Pripakhaylo, A.V.; Maryutina, T.A.; Shamsullin, A.I.; Ainullov, T.S. Role of Solvent Deasphalting in the Modern Oil Refining Practice and Trends in the Process Development. Russ. J. Appl. Chem. 2019, 92, 1634–1648. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Pakhmanova, O.A.; Kostyuk, A.V.; Antonov, S.V. Effect of the Asphaltene, Resin, and Wax Contents on the Physicochemical Properties and Quality Parameters of Crude Oils. Pet. Chem. 2017, 57, 1141–1143. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Strelets, L.A. Basic Fundamentals of Petroleum Rheology and Their Application for the Investigation of Crude Oils of Different Natures. Energy Fuels 2018, 32, 268–278. [Google Scholar] [CrossRef]
- Gray, M.R. Fundamentals of Partial Upgrading of Bitumen. Energy Fuels 2019, 33, 6843–6856. [Google Scholar] [CrossRef]
- Tchoukov, P.; Yang, F.; Xu, Z.; Dabros, T.; Czarnecki, J.; Sjöblom, J. Role of Asphaltenes in Stabilizing Thin Liquid Emulsion Films. Langmuir 2014, 30, 3024–3033. [Google Scholar] [CrossRef]
- Havre, T.E.; Sjöblom, J. Emulsion Stabilization by Means of Combined Surfactant Multilayer (D-Phase) and Asphaltene Particles. Colloids Surfaces A Physicochem. Eng. Asp. 2003, 228, 131–142. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Ilyin, S.O. Structure, Rheology and Possible Application of Water-in-Oil Emulsions Stabilized by Asphaltenes. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 618, 126442. [Google Scholar] [CrossRef]
- Qin, F.; Jiang, W.; Ni, G.; Wang, J.; Zuo, P.; Qu, S.; Shen, W. From Coal-Heavy Oil Co-Refining Residue to Asphaltene-Based Functional Carbon Materials. ACS Sustain. Chem. Eng. 2019, 7, 4523–4531. [Google Scholar] [CrossRef]
- Zuo, P.; Qu, S.; Shen, W. Asphaltenes: Separations, Structural Analysis and Applications. J. Energy Chem. 2019, 34, 186–207. [Google Scholar] [CrossRef]
- Bisheh, H.; Abdin, Y. Carbon Fibers: From PAN to Asphaltene Precursors; A State-of-Art Review. C 2023, 9, 19. [Google Scholar] [CrossRef]
- Saad, S.; Zeraati, A.S.; Roy, S.; Shahriar Rahman Saadi, M.A.; Radović, J.R.; Rajeev, A.; Miller, K.A.; Bhattacharyya, S.; Larter, S.R.; Natale, G.; et al. Transformation of Petroleum Asphaltenes to Carbon Fibers. Carbon 2022, 190, 92–103. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Gorbacheva, S.N.; Ilyin, S.O. The Deasphalting of Heavy Crude Oil by Silicone Oil Is a Green Method of Removing Heavy Compounds and Producing Bitumen. Geoenergy Sci. Eng. 2023, 227, 211940. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Vakhin, A.V.; Mikhailova, A.N.; Petrov, S.M.; Sitnov, S.A. Road Bitumen’s Based on the Vacuum Residue of Heavy Oil and Natural Asphaltite: Part II—Physical and Mechanical Properties. Pet. Sci. Technol. 2017, 35, 1687–1691. [Google Scholar] [CrossRef]
- Eshraghian, A.; Kamkar, M.; Sundararaj, U. Asphaltene/Polymer Composites: Morphology, Compatibility, and Rheological Properties. Can. J. Chem. Eng. 2023, 101, 1421–1439. [Google Scholar] [CrossRef]
- Wu, H.; Kessler, M.R. Asphaltene: Structural Characterization, Molecular Functionalization, and Application as a Low-Cost Filler in Epoxy Composites. RSC Adv. 2015, 5, 24264–24273. [Google Scholar] [CrossRef]
- Ding, R.; Torres, S.W.; Messman, J.; Bowen, D.E.; Bowler, N. Dynamics of Model Polycyclic Aromatic Hydrocarbon Compound-Epoxy Composites: A Dielectric Study. Polymer 2018, 136, 6–16. [Google Scholar] [CrossRef]
- Han, X.; Su, W.; Gong, J.; Xi, Z.; Zhang, J.; Cai, J.; Wang, Q.; Xie, H. Microstructure and Dynamic Mechanical Properties Epoxy/Asphaltene Composites. J. Therm. Anal. Calorim. 2022, 147, 2209–2219. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Kostyuk, A.V.; Kostina, J.V.; Bakhtin, D.S.; Makarova, V.V.; Antonov, S.V.; Ilyin, S.O. Heavy Crude Oil Asphaltenes as a Nanofiller for Epoxy Resin. Polym. Eng. Sci. 2020, 60, 1530–1545. [Google Scholar] [CrossRef]
- Miao, W.; Zhu, H.; Duan, T.; Chen, H.; Wu, F.; Jiang, L.; Wang, Z. High-Density Polyethylene Crystals with Double Melting Peaks Induced by Ultra-High-Molecular-Weight Polyethylene Fibre. R. Soc. Open Sci. 2018, 5, 180394. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.N.; Redhwi, H.H.; Younas, M.; Alghizzi, A.G.; Suliman, M.H.; Achilias, D.S. Effect of Natural Macromolecule Filler on the Properties of High-Density Polyethylene (HDPE). Macromol. Symp. 2018, 380, 1800072. [Google Scholar] [CrossRef]
- Minzagirova, A.M.; Gilmanova, A.R.; Borisova, Y.Y.; Borisov, D.N.; Galikhanov, M.F.; Ziganshin, M.A.; Yakubov, M.R. Polyolefin Composition Materials Filled with Oil Asphaltenes and their Functionalized Derivatives. J. Sib. Fed. Univ. Chem. 2020, 13, 408–417. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Younas, M.; Hussain, S.; Achilias, D.S. Use of Asphaltene Filler to Improve Low-Density Polyethylene Properties. Pet. Sci. Technol. 2018, 36, 756–764. [Google Scholar] [CrossRef]
- Moradkhani, R.; Hosseini-Dastgerdi, Z.; Sirousazar, M. High-Density Polyethylene/Asphaltene Composites: Thermal, Mechanical and Morphological Properties. Polym. Polym. Compos. 2021, 29, 1528–1533. [Google Scholar] [CrossRef]
- Siddiqui, M.N. Preparation and Properties of Polypropylene-Asphaltene Composites. Polym. Compos. 2017, 38, 1957–1963. [Google Scholar] [CrossRef]
- Melekhina, V.Y.; Kostyuk, A.V.; Smirnova, N.M.; Ilyin, S.O. Asphaltene-Stabilized Polyisobutylene Pressure-Sensitive Adhesives for Ultraviolet Protection and Surface Bonding. Materials 2023, 16, 1209. [Google Scholar] [CrossRef]
- Siddiqui, M.N. Using Asphaltenes as Filler in Methyl Methacrylate Polymer Composites. Pet. Sci. Technol. 2016, 34, 253–259. [Google Scholar] [CrossRef]
- Siddiqui, M.N. Studies of Different Properties of Polystyrene-Asphaltene Composites. Macromol. Symp. 2015, 354, 184–190. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Antonov, S.V.; Kostyuk, A.V.; Smirnova, N.M.; Makarova, V.V.; Ilyin, S.O. Composites Based on Polystyrene and Asphaltenes. Russ. J. Appl. Chem. 2019, 92, 1712–1717. [Google Scholar] [CrossRef]
- Wu, H.; Thakur, V.K.; Kessler, M.R. Novel Low-Cost Hybrid Composites from Asphaltene/SBS Tri-Block Copolymer with Improved Thermal and Mechanical Properties. J. Mater. Sci. 2016, 51, 2394–2403. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Kostyuk, A.V.; Smirnova, N.M.; Antonov, S.V.; Ilyin, S.O. Asphaltenes as a Tackifier for Hot-melt Adhesives Based on the Styrene-isoprene-styrene Block Copolymer. Polym. Eng. Sci. 2020, 60, 2224–2234. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Melekhina, V.Y.; Kostyuk, A.V.; Smirnova, N.M. Hot-Melt and Pressure-Sensitive Adhesives Based on Styrene-Isoprene-Styrene Triblock Copolymer, Asphaltene/Resin Blend and Naphthenic Oil. Polymers 2022, 14, 4296. [Google Scholar] [CrossRef] [PubMed]
- Overton, E.B.; Sharp, W.D.; Roberts, P. Toxicity of Petroleum. In Basic Environmental Toxicology; Cockerham, L.G., Shane, B.S., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 133–156. ISBN 978-1-315-13809-1. [Google Scholar]
- Schreiner, C.A. Review of Mechanistic Studies Relevant to the Potential Carcinogenicity of Asphalts. Regul. Toxicol. Pharmacol. 2011, 59, 270–284. [Google Scholar] [CrossRef]
- Borisova, Y.Y.; Minzagirova, A.M.; Gilmanova, A.R.; Galikhanov, M.F.; Borisov, D.N.; Yakubov, M.R. Heavy Oil Residues: Application as a Low-Cost Filler in Polymeric Materials. Civ. Eng. J. 2019, 5, 2554–2568. [Google Scholar] [CrossRef]
- Evdokimov, I.N.; Fesan, A.A.; Losev, A.P. Asphaltenes: Absorbers and Scatterers at Near-Ultraviolet–Visible–Near-Infrared Wavelengths. Energy Fuels 2017, 31, 3878–3884. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Younas, M.; Al-Arfaj, A.A.; Hussain, S.; Naim, M. Durability Study of Asphaltene-Reinforced HDPE and LDPE Composites under UV Irradiation and Local Weathering Exposure. Polym. Bull. 2021, 78, 4487–4503. [Google Scholar] [CrossRef]
- Baumhardt-Neto, R.; De Paoli, M.-A. Mechanical Degradation of Polypropylene: Effect of UV Irradiation. Polym. Degrad. Stab. 1993, 40, 59–64. [Google Scholar] [CrossRef]
- Tidjani, A. Photooxidation of Polypropylene under Natural and Accelerated Weathering Conditions. J. Appl. Polym. Sci. 1997, 64, 2497–2503. [Google Scholar] [CrossRef]
- Kotek, J.; Kelnar, I.; Baldrian, J.; Raab, M. Structural Transformations of Isotactic Polypropylene Induced by Heating and UV Light. Eur. Polym. J. 2004, 40, 2731–2738. [Google Scholar] [CrossRef]
- Cui, Q.; Yang, X.; Li, J.; Miao, Y.; Zhang, X. Microplastics Generation Behavior of Polypropylene Films with Different Crystalline Structures under UV Irradiation. Polym. Degrad. Stab. 2022, 199, 109916. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Ignatenko, V.Y.; Kostyuk, A.V.; Levin, I.S.; Bondarenko, G.N. Deasphalting of Heavy Crude Oil by Hexamethyldisiloxane: The Effect of a Solvent/Oil Ratio on the Structure, Composition, and Properties of Precipitated Asphaltenes. J. Pet. Sci. Eng. 2022, 208, 109329. [Google Scholar] [CrossRef]
- Raymont, J. Life After the Honeymoon: Getting to Know, Understand, Respect and Live with Your UV System and Process. SGIA J. 2001, 3, 29–39. [Google Scholar]
- Ilyin, S.; Kulichikhin, V.; Malkin, A. Characterization of Material Viscoelasticity at Large Deformations. Appl. Rheol. 2014, 24, 13653. [Google Scholar] [CrossRef]
- Schramm, G.A. Practical Approach to Rheology and Rheometry; Haake: Karlsruhe, Germany, 1994. [Google Scholar]
- Yadykova, A.Y.; Ilyin, S.O. Rheological and Adhesive Properties of Nanocomposite Bitumen Binders Based on Hydrophilic or Hydrophobic Silica and Modified with Bio-Oil. Constr. Build. Mater. 2022, 342, 127946. [Google Scholar] [CrossRef]
- Speight, J.G. The Chemistry and Technology of Petroleum, 5th ed.; Chemical Industries; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4398-7389-2. [Google Scholar]
- Demirbas, A.; Taylan, O. Removing of Resins from Crude Oils. Pet. Sci. Technol. 2016, 34, 771–777. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Nasyrova, Z.R.; Mikhailova, A.N.; Kosachev, I.P.; Aliev, F.A.; Vakhin, A.V. Composition of Oil after Hydrothermal Treatment of Cabonate-Siliceous and Carbonate Domanic Shale Rocks. Processes 2021, 9, 1798. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Strelets, L.A.; Ilyin, S.O. Infrared Spectral Classification of Natural Bitumens for Their Rheological and Thermophysical Characterization. Molecules 2023, 28, 2065. [Google Scholar] [CrossRef]
- Sowinski, P.; Piorkowska, E.; Boyer, S.A.E.; Haudin, J.-M.; Zapala, K. The Role of Nucleating Agents in High-Pressure-Induced Gamma Crystallization in Isotactic Polypropylene. Colloid. Polym. Sci. 2015, 293, 665–675. [Google Scholar] [CrossRef]
- Housmans, J.-W.; Gahleitner, M.; Peters, G.W.M.; Meijer, H.E.H. Structure–Property Relations in Molded, Nucleated Isotactic Polypropylene. Polymer 2009, 50, 2304–2319. [Google Scholar] [CrossRef]
- Huy, T.A.; Adhikari, R.; Lüpke, T.; Henning, S.; Michler, G.H. Molecular Deformation Mechanisms of Isotactic Polypropylene in α- and β-Crystal Forms by FTIR Spectroscopy: Molecular Deformation of α-/β-Crystal IPP. J. Polym. Sci. B Polym. Phys. 2004, 42, 4478–4488. [Google Scholar] [CrossRef]
- Goikhman, A.S.; Kirichenko, V.I.; Budnitskii, G.A.; Korolenko, M.P.; Matsibora, N.P. X-ray Diffraction Measurements of the Crystallinity of Polypropylene Fibres. Polym. Sci. USSR 1984, 26, 974–981. [Google Scholar] [CrossRef]
- García, M.D.C. Crude Oil Wax Crystallization. The Effect of Heavy n-Paraffins and Flocculated Asphaltenes. Energy Fuels 2000, 14, 1043–1048. [Google Scholar] [CrossRef]
- Ilyina, S.O.; Vlasova, A.V.; Gorbunova, I.Y.; Lukashov, N.I.; Kerber, M.L.; Ilyin, S.O. Epoxy Phase-Change Materials Based on Paraffin Wax Stabilized by Asphaltenes. Polymers 2023, 15, 3243. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Bagrov, V.V.; Komarov, P.D.; Ilyin, S.O.; Ivchenko, P.V. The Use of Branching Agents in the Synthesis of PBAT. Polymers 2022, 14, 1720. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sadrtdinova, G.I.; Komarov, P.D.; Minyaev, M.E.; Ilyin, S.O.; Kiselev, A.V.; Samurganova, T.I.; Ivchenko, P.V. Synthesis, Molecular Structure and Catalytic Performance of Heterocycle-Fused Cyclopentadienyl-Amido CGC of Ti (IV) in Ethylene (Co)Polymerization: The Formation and Precision Rheometry of Long-Chain Branched Polyethylenes. Eur. Polym. J. 2022, 176, 111397. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kulichikhin, V.G. Rheology and Miscibility of Linear/Hyperbranched Polydimethylsiloxane Blends and an Abnormal Decrease in Their Viscosity. Macromolecules 2023, 56, 6818–6833. [Google Scholar] [CrossRef]
- Colby, R.H.; Fetters, L.J.; Graessley, W.W. The Melt Viscosity-Molecular Weight Relationship for Linear Polymers. Macromolecules 1987, 20, 2226–2237. [Google Scholar] [CrossRef]
- Carriere, C.J. Evaluation of the Entanglement Molecular Weights of Maize Starches from Solution Rheological Measurements. Cereal Chem. 1998, 75, 360–364. [Google Scholar] [CrossRef]
- Niu, H.; Wang, Y.; Liu, X.; Wang, Y.; Li, Y. Determination of Plateau Moduli and Entanglement Molecular Weights of Ultra-High Molecular Weight Isotactic Polypropylene Synthesized by Ziegler-Natta Catalyst. Polym. Test. 2017, 60, 260–265. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Malkin, A.Y.; Kulichikhin, V.G.; Shaulov, A.Y.; Stegno, E.V.; Berlin, A.A.; Patlazhan, S.A. Rheological Properties of Polyethylene/Metaboric Acid Thermoplastic Blends. Rheol. Acta 2014, 53, 467–475. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Gorbacheva, S.N.; Yadykova, A.Y. Rheology and Tribology of Nanocellulose-Based Biodegradable Greases: Wear and Friction Protection Mechanisms of Cellulose Microfibrils. Tribol. Int. 2023, 178, 108080. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Ilyin, S.O.; Arinina, M.P.; Kulichikhin, V.G. The Rheological State of Suspensions in Varying the Surface Area of Nano-Silica Particles and Molecular Weight of the Poly(Ethylene Oxide) Matrix. Colloid. Polym. Sci. 2017, 295, 555–563. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Ilyin, S.O. Bitumen Improvement with Bio-Oil and Natural or Organomodified Montmorillonite: Structure, Rheology, and Adhesion of Composite Asphalt Binders. Constr. Build. Mater. 2023, 364, 129919. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Ilyin, S.O. Nanocellulose-Stabilized Bitumen Emulsions as a Base for Preparation of Nanocomposite Asphalt Binders. Carbohydr. Polym. 2023, 313, 120896. [Google Scholar] [CrossRef] [PubMed]
- Ilyin, S.O.; Malkin, A.Y.; Kulichikhin, V.G. Application of Large Amplitude Oscillatory Shear for the Analysis of Polymer Material Properties in the Nonlinear Mechanical Behavior. Polym. Sci. Ser. A 2014, 56, 98–110. [Google Scholar] [CrossRef]
- Hyun, K.; Wilhelm, M.; Klein, C.O.; Cho, K.S.; Nam, J.G.; Ahn, K.H.; Lee, S.J.; Ewoldt, R.H.; McKinley, G.H. A Review of Nonlinear Oscillatory Shear Tests: Analysis and Application of Large Amplitude Oscillatory Shear (LAOS). Prog. Polym. Sci. 2011, 36, 1697–1753. [Google Scholar] [CrossRef]
- Li, D.; Xin, Y.; Song, Y.; Dong, T.; Ben, H.; Yu, R.; Han, G.; Zhang, Y. Crystalline Modification of Isotactic Polypropylene with a Rare Earth Nucleating Agent Based on Ultrasonic Vibration. Polymers 2019, 11, 1777. [Google Scholar] [CrossRef]
- Li, J.X.; Cheung, W.L.; Jia, D. A Study on the Heat of Fusion of β-Polypropylene. Polymer 1999, 40, 1219–1222. [Google Scholar] [CrossRef]
- Ruetsch, S.B.; Kamath, Y.; Weigmann, H.-D. Photodegradation of Human Hair: A Microscopy Study. In Comprehensive Series in Photosciences; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 175–205. ISBN 978-0-444-50839-3. [Google Scholar] [CrossRef]
- Chin, J.W. Durability of Composites Exposed to Ultraviolet Radiation. In Durability of Composites for Civil Structural Applications; Elsevier: Amsterdam, The Netherlands, 2007; pp. 80–97. ISBN 978-1-84569-035-9. [Google Scholar] [CrossRef]
- Searle, N.D. Environmental Effects on Polymeric Materials. In Plastics and the Environment; Andrady, A.L., Ed.; Wiley: Hoboken, NJ, USA, 2003; pp. 311–358. ISBN 978-0-471-09520-0. [Google Scholar] [CrossRef]
- Vaughan, G.B.; Tynan, E.C.; Yen, T.F. Vanadium Complexes and Porphyrins in Asphaltene, 2. The Nature of Highly Aromatic Substituted Porphins and Their Vanadyl Chelates. Chem. Geol. 1970, 6, 203–219. [Google Scholar] [CrossRef]
- White, J.R.; Turnbull, A. Weathering of Polymers: Mechanisms of Degradation and Stabilization, Testing Strategies and Modelling. J. Mater. Sci. 1994, 29, 584–613. [Google Scholar] [CrossRef]






| wasp, wt% | Before UV | After UV |
|---|---|---|
| 0 | 44.1% (44.1%) 1 | 39.1% (39.1%) |
| 5 | 52.3% (55.1%) | 43.0% (45.3%) |
| 10 | 48.5% (53.9%) | 43.4% (48.2%) |
| 20 | 52.5% (65.6%) | 41.3% (51.6%) |
| 30 | 50.4% (72.0%) | 43.6% (62.2%) |
| wasp, wt% | UV | Tm, °C | ΔHm, J/g | Tcr, °C | ΔHcr, J/g | NDC, % |
|---|---|---|---|---|---|---|
| 0 | before | 166.6 | 114.9 | 112.7 | 121.3 | 100 |
| 5 | before | 166.1 | 103.4 | 114.2 | 106.2 | 93.4 |
| 10 | before | 166.2 | 87.7 | 112.0 | 105.0 | 90.6 |
| 20 | before | 166.0 | 81.3 | 110.9 | 92.5 | 92.0 |
| 30 | before | 164.3 | 67.2 | 111.6 | 87.4 | 93.5 |
| 0 | after | 161.5 | 75.4 | 99.3 | 159.2 | 99.3 |
| 5 | after | 164.8 | 92.2 | 104.1 | 105.2 | 88.0 |
| 10 | after | 165.8 | 85.5 | 104.7 | 104.4 | 89.3 |
| 20 | after | 167.5 | 81.8 | 105.1 | 98.1 | 95.2 |
| 30 | after | 165.4 | 66.8 | 104.7 | 81.9 | 89.9 |
| wasp, wt% | σ0, MPa | σUV, MPa | E0, MPa | EUV, MPa |
|---|---|---|---|---|
| 0 | 28 ± 2 | 1.8 ± 0.4 | 520 ± 80 | 13 ± 14 |
| 5 | 28 ± 4 | 4.2 ± 1.1 | 470 ± 100 | 50 ± 15 |
| 10 | 26 ± 4 | 6.1 ± 1.5 | 410 ± 130 | 50 ± 50 |
| 20 | 22 ± 5 | 9.9 ± 2.3 | 250 ± 150 | 100 ± 50 |
| 30 | 12 ± 2 | 6.5 ± 1.6 | 380 ± 70 | 120 ± 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melekhina, V.Y.; Vlasova, A.V.; Ilyin, S.O. Asphaltenes from Heavy Crude Oil as Ultraviolet Stabilizers against Polypropylene Aging. Polymers 2023, 15, 4313. https://doi.org/10.3390/polym15214313
Melekhina VY, Vlasova AV, Ilyin SO. Asphaltenes from Heavy Crude Oil as Ultraviolet Stabilizers against Polypropylene Aging. Polymers. 2023; 15(21):4313. https://doi.org/10.3390/polym15214313
Chicago/Turabian StyleMelekhina, Viktoria Y., Anna V. Vlasova, and Sergey O. Ilyin. 2023. "Asphaltenes from Heavy Crude Oil as Ultraviolet Stabilizers against Polypropylene Aging" Polymers 15, no. 21: 4313. https://doi.org/10.3390/polym15214313