Tuning of Morphological and Antibacterial Properties of Poly(3,4-ethylenedioxythiophene):Peroxodisulfate by Methyl Violet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of PEDOT with or without MV Dye
2.3. Characterization
3. Results and Discussion
3.1. Morphology
3.2. Optical Properties
3.3. MALDI TOF Spectra
3.4. Yield and Conductivity
3.5. Thermogravimetric Analysis
3.6. Raman Spectroscopy
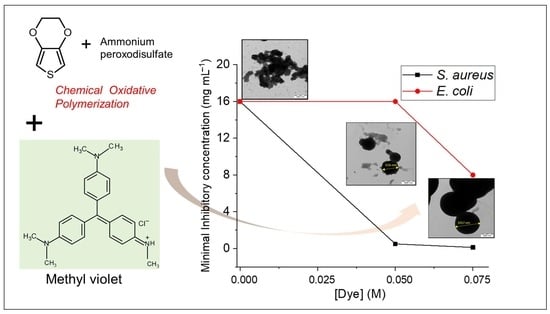
3.7. Minimal Inhibitory Concentration
3.8. Cyclic Voltammetry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cha, B.G.; Lee, D.; Kim, T.; Piao, Y.; Kim, J. Iron Oxide@Polypyrrole Core-Shell Nanoparticles as the Platform for Photothermal Agent and Electrochemical Biosensor. J. Nanosci. Nanotechnol. 2016, 16, 6942–6948. [Google Scholar] [CrossRef]
- Šetka, M.; Drbohlavová, J.; Hubálek, J. Nanostructured Polypyrrole-Based Ammonia and Volatile Organic Compound Sensors. Sensors 2017, 17, 562. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Chang, H.C.; Jefferson, S.; Daniels, D.E. Application of Conductive Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) (PEDOT: PSS) Polymers in Potential Biomedical Engineering. J. Pharm. Investig. 2020, 50, 437–444. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Wang, M.; Yang, Z.; Lin, L.; Xiong, Y.; Xu, Z.; Wang, J. H2O2/near-Infrared Light-Responsive Nanotheronostics for MRI-Guided Synergistic Chemo/Photothermal Cancer Therapy. Nanomedicine 2019, 14, 2189–2207. [Google Scholar] [CrossRef]
- Woeppel, K.M.; Zheng, X.S.; Schulte, Z.M.; Rosi, N.L.; Cui, X.T. Nanoparticle Doped PEDOT for Enhanced Electrode Coatings and Drug Delivery. Adv. Healthc. Mater. 2019, 8, 1900622. [Google Scholar] [CrossRef] [PubMed]
- Babaie, A.; Bakhshandeh, B.; Abedi, A.; Mohammadnejad, J.; Shabani, I.; Ardeshirylajimi, A.; Moosavi, S.; Amini, J.; Tayebi, L. Synergistic Effects of Conductive PVA/PEDOT Electrospun Scaffolds and Electrical Stimulation for More Effective Neural Tissue Engineering. Eur. Polym. J. 2020, 140, 110051. [Google Scholar] [CrossRef]
- Mahira, S.; Jain, A.; Khan, W.; Domb, A.J. Antimicrobial Materials—An Overview. In Antimicrobial Materials for Biomedical Applications; Royal Society of Chemistry: London, UK, 2019; pp. 1–37. [Google Scholar]
- Sedighi, A.; Montazer, M.; Mazinani, S. Fabrication of Electrically Conductive Superparamagnetic Fabric with Microwave Attenuation, Antibacterial Properties and UV Protection Using PEDOT/Magnetite Nanoparticles. Mater. Des. 2018, 160, 34–47. [Google Scholar] [CrossRef]
- Kiefer, R.; Lee, R.J.; Temmer, R.; Tamm, T.; Aabloo, A. Chitosan Combined with Conducting Polymers for Novel Functionality: Antioxidant and Antibacterial Activity. Key Eng. Mater. 2014, 605, 428–431. [Google Scholar] [CrossRef]
- Wan, C.; Li, J. Cellulose Aerogels Functionalized with Polypyrrole and Silver Nanoparticles: In-Situ Synthesis, Characterization and Antibacterial Activity. Carbohydr. Polym. 2016, 146, 362–367. [Google Scholar] [CrossRef]
- Mansour Lakourj, M.; Norouzian, R.S.; Esfandyar, M.; Ghasemi Mir, S. Conducting Nanocomposites of Polypyrrole-Co-Polyindole Doped with Carboxylated CNT: Synthesis Approach and Anticorrosion/Antibacterial/Antioxidation Property. Mater. Sci. Eng. B 2020, 261, 114673. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Ryskevic, N.; Oztekin, Y.; Kausaite-Minkstimiene, A.; Jursenas, S.; Baniukevic, J.; Kirlyte, J.; Bubniene, U.; Ramanaviciene, A. Immunosensor Based on Fluorescence Quenching Matrix of the Conducting Polymer Polypyrrole. Anal. Bioanal. Chem. 2010, 398, 3105–3113. [Google Scholar] [CrossRef]
- Ayranci, R.; Kirbay, F.O.; Demirkol, D.O.; Ak, M.; Timur, S. Copolymer Based Multifunctional Conducting Polymer Film for Fluorescence Sensing of Glucose. Methods Appl. Fluoresc. 2018, 6, 035012. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.B.; Yslas, E.I.; Rivarola, C.R.; Barbero, C.A. Synthesis of Polyaniline (PANI) and Functionalized Polyaniline (F-PANI) Nanoparticles with Controlled Size by Solvent Displacement Method. Application in Fluorescence Detection and Bacteria Killing by Photothermal Effect. Nanotechnology 2018, 29, 125604. [Google Scholar] [CrossRef]
- Bartel, M.; Wysocka, B.; Krug, P.; Kępińska, D.; Kijewska, K.; Blanchard, G.J.; Kaczyńska, K.; Lubelska, K.; Wiktorska, K.; Głowala, P.; et al. Magnetic Polymer Microcapsules Loaded with Nile Red Fluorescent Dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 195, 148–156. [Google Scholar] [CrossRef]
- Groenendaal, L.B.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future. Adv. Mater. 2000, 12, 7. [Google Scholar] [CrossRef]
- Rossetti, N.; Luthra, P.; Hagler, J.; Jae Lee, A.H.; Bodart, C.; Li, X.; Ducharme, G.; Soavi, F.; Amilhon, B.; Cicoira, F. Poly(3,4-ethylenedioxythiophene) (PEDOT) Coatings for High-Quality Electromyography Recording. ACS Appl. Bio Mater. 2019, 2, 5154–5163. [Google Scholar] [CrossRef] [PubMed]
- Bolin, M.H.; Svennersten, K.; Wang, X.; Chronakis, I.S.; Richter-Dahlfors, A.; Jager, E.W.H.; Berggren, M. Nano-Fiber Scaffold Electrodes Based on PEDOT for Cell Stimulation. Sens. Actuators B Chem. 2009, 142, 451–456. [Google Scholar] [CrossRef]
- Flampouri, E.; Kintzios, S. Nafion and Polylysine Treated PEDOT Mammalian Cell Biosensor. Procedia Eng. 2011, 25, 976–979. [Google Scholar] [CrossRef]
- Tian, H.C.; Liu, J.Q.; Kang, X.Y.; Wei, D.X.; Zhang, C.; Du, J.C.; Yang, B.; Chen, X.; Yang, C.S. Biotic and Abiotic Molecule Dopants Determining the Electrochemical Performance, Stability and Fibroblast Behavior of Conducting Polymer for Tissue Interface. RSC Adv. 2014, 4, 47461–47471. [Google Scholar] [CrossRef]
- Kiristi, M.; Oksuz, A.U.; Oksuz, L.; Ulusoy, S. Electrospun Chitosan/PEDOT Nanofibers. Mater. Sci. Eng. C 2013, 33, 3845–3850. [Google Scholar] [CrossRef] [PubMed]
- Triguero, J.; Zanuy, D.; Alemán, C. Impact of Protein-Polymer Interactions in the Antimicrobial Activity of Lysozyme/Poly(3,4-ethylenedioxythiophene) Biocapacitors. ChemistrySelect 2018, 3, 9714–9724. [Google Scholar] [CrossRef]
- Minisy, I.M.; Acharya, U.; Kobera, L.; Trchová, M.; Unterweger, C.; Breitenbach, S.; Brus, J.; Pfleger, J.; Stejskal, J.; Bober, P. Highly Conducting 1-D Polypyrrole Prepared in the Presence of Safranin. J. Mater. Chem. C 2020, 8, 12140–12147. [Google Scholar] [CrossRef]
- Gupta, S.; Acharya, U.; Pištěková, H.; Taboubi, O.; Morávková, Z.; Kašparová, M.; Humpolíček, P.; Bober, P. Tuning the Conductivity, Morphology, and Capacitance with Enhanced Antibacterial Properties of Polypyrrole by Acriflavine Hydrochloride. ACS Appl. Polym. Mater. 2021, 3, 6063–6069. [Google Scholar] [CrossRef]
- Bober, P.; Li, Y.; Acharya, U.; Panthi, Y.; Pfleger, J.; Humpolíček, P.; Trchová, M.; Stejskal, J. Acid Blue Dyes in Polypyrrole Synthesis: The Control of Polymer Morphology at Nanoscale in the Promotion of High Conductivity and the Reduction of Cytotoxicity. Synth. Met. 2018, 237, 40–49. [Google Scholar] [CrossRef]
- Patir, A.; Hwang, G.B.; Lourenco, C.; Nair, S.P.; Carmalt, C.J.; Parkin, I.P. Crystal Violet-Impregnated Slippery Surface to Prevent Bacterial Contamination of Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 5478–5485. [Google Scholar] [CrossRef]
- Paradee, N.; Sirivat, A. Synthesis of Poly(3,4-ethylenedioxythiophene) Nanoparticles via Chemical Oxidation Polymerization. Polym. Int. 2014, 63, 106–113. [Google Scholar] [CrossRef]
- Bai, M.; Wang, X.; Li, B. Capacitive Behavior and Material Characteristics of Congo Red Doped Poly (3,4-Ethylene Dioxythiophene). Electrochim. Acta 2018, 283, 590–596. [Google Scholar] [CrossRef]
- Dai, T.; Lu, Y. Water-Soluble Methyl Orange Fibrils as Versatile Templates for the Fabrication of Conducting Polymer Microtubules. Macromol. Rapid Commun. 2007, 28, 629–633. [Google Scholar] [CrossRef]
- Gupta, S.; Patra, A. Facile Polymerization Method for Poly(3,4-ethylenedioxythiophene) and Related Polymers Using Iodine Vapour. New J. Chem. 2020, 44, 6883–6888. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, A.; Kumar, R.; Patra, A. Solid-State Synthesis of Conjugated Doped Poly(3,4-ethylenedioxythiophene): An Effective Adsorbent for Selective Anionic Dye Removal. React. Funct. Polym. 2021, 165, 104972. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, T. Study of Modified PEDOT: PSS for Tuning the Optical Properties of Its Conductive Thin Films. J. Sci. Adv. Mater. Devices 2019, 4, 538–543. [Google Scholar] [CrossRef]
- Chandraboss, V.L.; Kamalakkannan, J.; Prabha, S.; Senthilvelan, S. An Efficient Removal of Methyl Violet from Aqueous Solution by an AC-Bi/ZnO Nanocomposite Material. RSC Adv. 2015, 5, 25857–25869. [Google Scholar] [CrossRef]
- Kochervinskii, V.V.; Gradova, M.A.; Gradov, O.V.; Kiselev, D.A.; Ilina, T.S.; Kalabukhova, A.V.; Kozlova, N.V.; Shmakova, N.A.; Bedin, S.A. Structural, Optical, and Electrical Properties of Ferroelectric Copolymer of Vinylidenefluoride Doped with Rhodamine 6G Dye. J. Appl. Phys. 2019, 125, 044103. [Google Scholar] [CrossRef]
- Ryu, N.; Okazaki, Y.; Pouget, E.; Takafuji, M.; Nagaoka, S.; Ihara, H.; Oda, R. Fluorescence emission originated from the H-aggregated cyanine dye with chiral gemini surfactant assemblies having a narrow absorption band and a remarkably large Stokes shift. Chem. Commun. 2017, 53, 8870–8873. [Google Scholar] [CrossRef]
- Hestand, N.J.; Spano, F.C. Expanded theory of H-and J-molecular aggregates: The effects of vibronic coupling and intermolecular charge transfer. Chem. Rev. 2018, 118, 7069–7163. [Google Scholar] [CrossRef]
- Sun, H.; Lu, B.Y.; Duan, X.M.; Jingkun Xu, J.K.; Dong, L.; Zhu, X.F.; Zhang, K.X.; Hu, D.F.; Ming, S.L. Electrosynthesis and Characterization of a New Conducting Copolymer from 2′-aminomethyl-3,4-ethylenedioxythiophene and 3,4-ethylenedioxythiophene. Int. J. Electrochem. Sci. 2015, 10, 3236–3249. [Google Scholar] [CrossRef]
- Shahinyan, G.A.; Amirbekyan, A.Y.; Markarian, S.A. Photophysical Properties of Methylene Blue in Water and in Aqueous Solutions of Dimethylsulfoxide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 217, 170–175. [Google Scholar] [CrossRef]
- Elschner, A.; Kirchmeyer, S.; Lövenich, W.; Merker, U.; Reuter, K. PEDOT: Principles and Applications of an Intrinsically Conductive Polyme; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–380. [Google Scholar]
- Stejskal, J. Interaction of Conducting Polymers, Polyaniline and Polypyrrole, with Organic Dyes: Polymer Morphology Control, Dye Adsorption and Photocatalytic Decomposition. Chem. Pap. 2019, 74, 1–54. [Google Scholar] [CrossRef]
- Bober, P.; Zasonska, B.A.; Humpolíček, P.; Kuceková, Z.; Varga, M.; Horák, D.; Babayan, V.; Kazantseva, N.; Prokeš, J.; Stejskal, J. Polyaniline–Maghemite Based Dispersion: Electrical, Magnetic Properties and Their Cytotoxicity. Synth. Met. 2016, 214, 23–29. [Google Scholar] [CrossRef]
- Lapides, I.; Yariv, S.; Golodnitsky, D. Simultaneous DTA-TG study of montmorillonite mechanochemically treated with crystal-violet. J. Therm. Anal. Calorim. 2002, 67, 99–112. [Google Scholar] [CrossRef]
- Gupta, S.; Taboubi, O.; Acharya, U.; Lhotka, M.; Pokorný, V.; Morávková, Z.; Hromádková, J.; Bober, P. Nanostructured poly (N-methyl pyrrole) with enhanced conductivity and capacitance. Synth Met. 2022, 290, 117134. [Google Scholar] [CrossRef]
- Garreau, S.; Louarn, G.; Buisson, J.P.; Froyer, G.; Lefrant, S. In Situ Spectroelectrochemical Raman Studies of Poly(3,4-ethylenedioxythiophene) (PEDT). Macromolecules 1999, 32, 6807–6812. [Google Scholar] [CrossRef]
- Zanfrognini, B.; Colina, A.; Heras, A.; Zanardi, C.; Seeber, R.; López-Palacios, J. A UV–Visible/Raman Spectroelectrochemical Study of the Stability of Poly(3,4-ethylendioxythiophene) Films. Polym. Degrad. Stab. 2011, 96, 2112–2119. [Google Scholar] [CrossRef]
- Szkoda, M.; Nowaczyk, G.; Lisowska-Oleksiak, A.; Siuzdak, K. The Influence of Polarization of Titania Nanotubes Modified by a Hybrid System Made of a Conducting Polymer PEDOT and Prussian Blue Redox Network on the Raman Spectroscopy Response and Photoelectrochemical Properties. Electrochim. Acta 2018, 279, 34–43. [Google Scholar] [CrossRef]
- Almeida, P.V.; Izumi, C.M.S.; Dos Santos, H.F.; SantAna, A.C. Spectroscopic Characterization of PEDOT: PSS Conducting Polymer by Resonance Raman and SERRS Spectroscopies. Quim. Nova 2019, 42, 1073–1080. [Google Scholar]
- Sakmeche, N.; Aaron, J.J.; Fall, M.; Aeiyach, S.; Jouini, M.; Lacroix, J.C.; Lacaze, P.C. Anionic Micelles; a New Aqueous Medium for Electropolymerization of Poly(3,4-ethylenedioxythiophene) Films on Pt Electrodes. Chem. Commun. 1996, 24, 2723–2724. [Google Scholar] [CrossRef]
- Lisowska-Oleksiak, A.; Nowak, A.P.; Wilamowska, M.; Sikora, M.; Szczerba, W.; Kapusta, C. Ex Situ XANES, XPS and Raman Studies of Poly(3,4-Ethylenedioxythiophene) Modified by Iron Hexacyanoferrate. Synth. Met. 2010, 160, 1234–1240. [Google Scholar] [CrossRef]
- Chiu, W.W.; Travaš-Sejdić, J.; Cooney, R.P.; Bowmaker, G.A. Studies of Dopant Effects in Poly(3,4-ethylenedi-oxythiophene) Using Raman Spectroscopy. J. Raman Spectrosc. 2006, 37, 1354–1361. [Google Scholar] [CrossRef]
- Kvarnström, C.; Neugebauer, H.; Blomquist, S.; Ahonen, H.J.; Kankare, J.; Ivaska, A. In Situ Spectroelectrochemica Characterization of Poly(3,4-ethylenedioxythiophene). Electrochim. Acta 1999, 44, 2739–2750. [Google Scholar] [CrossRef]
- Gupta, S.; Datt, R.; Mishra, A.; Tsoi, W.C.; Patra, A.; Bober, P. Poly (3, 4-ethylenedioxythiophene): Poly (styrene sulfonate) in antibacterial, tissue engineering and biosensors applications: Progress, challenges and perspectives. J. Appl. Polym. Sci. 2022, 139, e52663. [Google Scholar] [CrossRef]
- Madhan Kumar, A.; Adesina, A.Y.; Hussein, M.A.; Ramakrishna, S.; Al-Aqeeli, N.; Akhtar, S.; Saravanan, S. PEDOT/FHA Nanocomposite Coatings on Newly Developed Ti-Nb-Zr Implants: Biocompatibility and Surface Protection against Corrosion and Bacterial Infections. Mater. Sci. Eng. C 2019, 98, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Stepanenko, V.; Dehm, V.; Prins, P.; Siebbeles, L.D.A.; Seibt, J.; Marquetand, P.; Engel, V.; Würthner, F. Photoluminescence and Conductivity of Self-Assembled π–π Stacks of Perylene Bisimide Dyes. Chem. A Eur. J. 2007, 13, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Zasońska, B.A.; Acharya, U.; Pfleger, J.; Humpolíček, P.; Vajďák, J.; Svoboda, J.; Petrovsky, E.; Hromádková, J.; Walterová, Z.; Bober, P. Multifunctional Polypyrrole@maghemite@silver Composites: Synthesis, Physico-Chemical Characterization and Antibacterial Properties. Chem. Pap. 2018, 72, 1789–1797. [Google Scholar] [CrossRef]













| [MV] (M) | Yield (g g−1) | Molar Fraction of MV in the Composite | Conductivity (S cm−1) |
|---|---|---|---|
| 0 | 0.77 | 0 | 1.7 × 10–3 |
| 0.005 | 0.67 | 0.0048 | 3.4 × 10–5 |
| 0.010 | 0.74 | 0.015 | 4 × 10–6 |
| 0.020 | 0.77 | 0.024 | 3.1 × 10–8 |
| 0.050 | 0.95 | 0.15 | 1.5 × 10–11 |
| 0.075 | 1.4 | 0.30 | 9.5 × 10–12 |
| Position | Assignment | Reference |
|---|---|---|
| 1568 | asymmetrical C=C stretching in doped units | [44,45] |
| 1530 | asymmetrical C=C stretching in neutral units | [44,46,47,48] |
| 1493 | asymmetrical C=C stretching in doped units | [44,45,49] |
| 1450 | symmetrical C=C stretching in doped units | [44,45,49,50] |
| 1440 | symmetrical C=C stretching in doped units | [44,45,49] |
| 1430 | symmetrical C=C stretching in neutral units | [44,46,48,49,50] |
| 1360 | symmetrical Cβ—Cβ stretching in neutral units | [44,45,46,47,48,49,50] |
| 1260 | symmetrical Cα—Cα′ stretching | [44,45,46,47,48,49] |
| 1235 | symmetrical Cα—Cα′ stretching in doped units and C—H deformation | [44,46,49] |
| 1132 | C—O stretching | [46,47,48,51] |
| 1095 | C—O—C deformation | [46,47,49,50] |
| 1077 | C—O—C deformation | [44,51] |
| 987 | oxyethylene ring | [45,46,47,49,50] |
| 943 | C—S deformation | [51] |
| 857 | oxyethylene ring, doped units | [44,47] |
| 700/708 | C—S—C symmetrical deformation | [45,46,47,48,49,50,51] |
| 575 | oxyethylene ring deformation | [45,46,47,49,50] |
| 435 | oxyethylene ring deformation | [46,47] |
| Bacteria | Minimum Inhibitory Concentration (mg mL−1) | |||||
|---|---|---|---|---|---|---|
| 0 M | 0.005 M | 0.01 M | 0.02 M | 0.05 M | 0.075 M | |
| S. aureus | 16 | >32 | >32 | >32 | 0.5 | 0.125 |
| E. coli | 16 | >32 | >32 | >32 | 16 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Acharya, U.; Thottappali, M.A.; Pištěková, H.; Morávková, Z.; Hromádková, J.; Taboubi, O.; Pfleger, J.; Humpolíček, P.; Bober, P. Tuning of Morphological and Antibacterial Properties of Poly(3,4-ethylenedioxythiophene):Peroxodisulfate by Methyl Violet. Polymers 2023, 15, 3026. https://doi.org/10.3390/polym15143026
Gupta S, Acharya U, Thottappali MA, Pištěková H, Morávková Z, Hromádková J, Taboubi O, Pfleger J, Humpolíček P, Bober P. Tuning of Morphological and Antibacterial Properties of Poly(3,4-ethylenedioxythiophene):Peroxodisulfate by Methyl Violet. Polymers. 2023; 15(14):3026. https://doi.org/10.3390/polym15143026
Chicago/Turabian StyleGupta, Sonal, Udit Acharya, Muhammed Arshad Thottappali, Hana Pištěková, Zuzana Morávková, Jiřina Hromádková, Oumayma Taboubi, Jiří Pfleger, Petr Humpolíček, and Patrycja Bober. 2023. "Tuning of Morphological and Antibacterial Properties of Poly(3,4-ethylenedioxythiophene):Peroxodisulfate by Methyl Violet" Polymers 15, no. 14: 3026. https://doi.org/10.3390/polym15143026