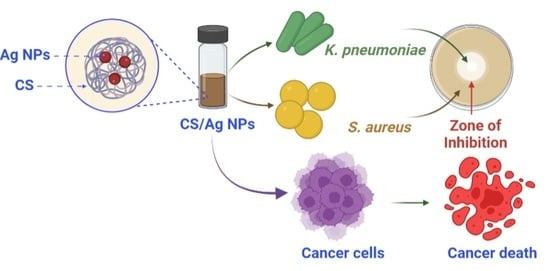
Facile Synthesis and Characterization of Chitosan Functionalized Silver Nanoparticles for Antibacterial and Anti-Lung Cancer Applications
Abstract
:1. Introduction
2. Experimental Sections
2.1. Chemicals and Cultures
2.2. Extraction of S. acuta Leaf Samples
2.3. Synthesis of CS/Ag NPs
2.4. Physico-Chemical Characterization
2.4.1. Optical Studies
2.4.2. FTIR
2.4.3. SEM-EDS
2.4.4. TEM
2.5. Disc Diffusion Antibacterial Activity Assay
2.6. AO/EtBr Dual Staining and Growth Curve Assay
2.7. Growth Curve Analysis
2.8. Anticancer MTT Assay against A549 Cells
2.9. Apoptosis Induction Studies
2.10. Statistical Analysis
3. Results and Discussion
3.1. Formation of CS/Ag NPs
3.2. Characterization Studies
3.2.1. Optical Studies
3.2.2. FTIR-Functional Groups Investigation
3.2.3. Microscopic and EDS Analysis
3.2.4. TEM Analysis
3.3. Disc Diffusion Bactericidal Assay
3.4. Dual Fluorescent Staining
3.5. Anticancer Studies
3.5.1. MTT Assay
3.5.2. AO/EtBr Fluorescent Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Hao, M.; Li, L.; Luo, Q.; Deng, S.; Yang, Y.; Liu, Y.; Fang, W.; Song, E. Research Progress of Contrast Agents for Bacterial Infection Imaging in Vivo. TrAC-Trends Anal. Chem. 2023, 159, 116916. [Google Scholar] [CrossRef]
- Tsikourkitoudi, V.; Henriques-Normark, B.; Sotiriou, G.A. Inorganic Nanoparticle Engineering against Bacterial Infections. Curr. Opin. Chem. Eng. 2022, 38, 100872. [Google Scholar] [CrossRef]
- Bharathi, D.; Rajamani, R.; Sibuh, B.Z.; Pandit, S.; Agrawal, S.; Mishra, N.; Sahni, M.; Thakur, V.K.; Gupta, P.K. Biogenic Preparation, Characterization, and Biomedical Applications of Chitosan Functionilized Iron Oxide Nanocomposite. J. Compo. Sci. 2022, 6, 120. [Google Scholar] [CrossRef]
- Mishra, A.; Pradhan, D.; Halder, J.; Biswasroy, P.; Rai, V.K.; Dubey, D.; Kar, B.; Ghosh, G.; Rath, G. Metal Nanoparticles against Multi-Drug-Resistance Bacteria. J. Inorg. Biochem. 2022, 237, 111938. [Google Scholar] [CrossRef]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.-S.; Yang, H.; Sun, L. Antimicrobial Peptides for Combating Drug-Resistant Bacterial Infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Li, X.; Liu, W. What Happens When Nanoparticles Encounter Bacterial Antibiotic Resistance? Sci. Total Environ. 2023, 876, 162856. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal Activity of Silver Nanoparticles: A Mechanistic Review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Malik, M.; Aamir Iqbal, M.; Iqbal, Y.; Malik, M.; Bakhsh, S.; Irfan, S.; Ahmad, R.; Pham, P.V. Biosynthesis of Silver Nanoparticles for Biomedical Applications: A Mini Review. Inorg. Chem. Commun. 2022, 145, 109980. [Google Scholar] [CrossRef]
- Tharani, S.; Bharathi, D.; Ranjithkumar, R. Extracellular Green Synthesis of Chitosan-Silver Nanoparticles Using Lactobacillus Reuteri for Antibacterial Applications. Biocatal. Agric. Biotechnol. 2020, 30, 101838. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Hossain, N.; Islam, M.A.; Chowdhury, M.A. Synthesis and Characterization of Plant Extracted Silver Nanoparticles and Advances in Dental Implant Applications. Heliyon 2022, 8, e12313. [Google Scholar] [CrossRef]
- Islam, M.A.; Jacob, M.V.; Antunes, E. A Critical Review on Silver Nanoparticles: From Synthesis and Applications to Its Mitigation through Low-Cost Adsorption by Biochar. J. Environ. Manage. 2021, 281, 111918. [Google Scholar] [CrossRef]
- Bharathi, D.; Josebin, M.D.; Vasantharaj, S.; Bhuvaneshwari, V. Biosynthesis of Silver Nanoparticles Using Stem Bark Extracts of Diospyros Montana and Their Antioxidant and Antibacterial Activities. J. Nanostructure Chem. 2018. [Google Scholar] [CrossRef] [Green Version]
- Seerangaraj, V.; Sathiyavimal, S.; Shankar, S.N.; Nandagopal, J.G.T.; Balashanmugam, P.; Al-Misned, F.A.; Shanmugavel, M.; Senthilkumar, P.; Pugazhendhi, A. Cytotoxic Effects of Silver Nanoparticles on Ruellia Tuberosa: Photocatalytic Degradation Properties against Crystal Violet and Coomassie Brilliant Blue. J. Environ. Chem. Eng. 2021, 9, 105088. [Google Scholar] [CrossRef]
- Ahmed, A.; Usman, M.; Ji, Z.; Rafiq, M.; Yu, B.; Shen, Y.; Cong, H. Nature-Inspired Biogenic Synthesis of Silver Nanoparticles for Antibacterial Applications. Mater. Today Chem. 2023, 27. [Google Scholar] [CrossRef]
- Heinemann, M.G.; Rosa, C.H.; Rosa, G.R.; Dias, D. Biogenic Synthesis of Gold and Silver Nanoparticles Used in Environmental Applications: A Review. Trends Environ. Anal. Chem. 2021, 30. [Google Scholar] [CrossRef]
- Ashique, S.; Upadhyay, A.; Hussain, A.; Bag, S.; Chaterjee, D.; Rihan, M.; Mishra, N.; Bhatt, S.; Puri, V.; Sharma, A.; et al. Green Biogenic Silver Nanoparticles, Therapeutic Uses, Recent Advances, Risk Assessment, Challenges, and Future Perspectives. J. Drug Deliv. Sci. Technol. 2022, 77, 103876. [Google Scholar] [CrossRef]
- Anshiba, J.; Poonkothai, M.; Karthika, P.; Mythili, R. Green Route to a Novel and Ecofriendly Phytosynthesis of Silver Nanoparticles Using Platycladus Orientalis L. Leaf Extract. Mater. Lett. 2022, 309, 131347. [Google Scholar] [CrossRef]
- Hai, N.D.; Dat, N.M.; Huong, L.M.; Tai, L.T.; Thinh, D.B.; Nam, N.T.H.; Dat, N.T.; Phong, M.T.; Hieu, N.H. Phytosynthesis of Silver Nanoparticles Using Mangifera Indica Leaves Extract at Room Temperature: Formation Mechanism, Catalytic Reduction, Colorimetric Sensing, and Antimicrobial Activity. Colloids Surfaces B Biointerfaces 2022, 220, 112974. [Google Scholar] [CrossRef]
- Suriyakala, G.; Sathiyaraj, S.; Devanesan, S.; AlSalhi, M.S.; Rajasekar, A.; Maruthamuthu, M.K.; Babujanarthanam, R. Phytosynthesis of Silver Nanoparticles from Jatropha Integerrima Jacq. Flower Extract and Their Possible Applications as Antibacterial and Antioxidant Agent. Saudi J. Biol. Sci. 2022, 29, 680–688. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Ekennia, A.C.; Ehiri, R.C.; Nnaji, N.J. Phytosynthesis of Silver Nanoparticles Using Aqueous Leaf Extracts of Lippia Citriodora: Antimicrobial, Larvicidal and Photocatalytic Evaluations. Mater. Sci. Eng. C 2017, 75, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Otari, S.V.; Patil, R.M.; Ghosh, S.J.; Pawar, S.H. Green Phytosynthesis of Silver Nanoparticles Using Aqueous Extract of Manilkara Zapota (L.) Seeds and Its Inhibitory Action against Candida Species. Mater. Lett. 2014, 116, 367–369. [Google Scholar] [CrossRef]
- Takcı, D.K.; Ozdenefe, M.S.; Genc, S. Green Synthesis of Silver Nanoparticles with an Antibacterial Activity Using Salvia Officinalis Aqueous Extract. J. Cryst. Growth 2023, 614, 127239. [Google Scholar] [CrossRef]
- Sharma, K.; Guleria, S.; Hussain, K.; Majeed, A.; Sharma, N.; Pawar, K.D.; Kumar, V.; Kumar, V. Photocatalytic and Biological Properties of Silver Nanoparticles Synthesized Using Callistemon Lanceolatus Leaf Extract. Ind. Crop. Prod. 2023, 202, 116951. [Google Scholar] [CrossRef]
- Pavan, S.R.; Venkatesan, J.; Prabhu, A. Anticancer Activity of Silver Nanoparticles from the Aqueous Extract of Dictyota Ciliolata on Non-Small Cell Lung Cancer Cells. J. Drug Deliv. Sci. Technol. 2022, 74, 103525. [Google Scholar] [CrossRef]
- Kamdoum, B.C.; Simo, I.; Wouamba, S.C.N.; Tchatat Tali, B.M.; Ngameni, B.; Fotso, G.W.; Ambassa, P.; Fabrice, F.B.; Lenta, B.N.; Sewald, N.; et al. Chemical Constituents of Two Cameroonian Medicinal Plants: Sida Rhombifolia L. and Sida Acuta Burm. f. (Malvaceae) and Their Antiplasmodial Activity. Nat. Prod. Res. 2022, 36, 5311–5318. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Gevrenova, R.; Sinan, K.I.; Bayarslan, A.U.; Altunoglu, Y.C.; Zheleva-Dimitrova, D.; Ak, G.; Baloglu, M.C.; Etienne, O.K.; Lobine, D.; et al. New Perspectives into the Chemical Characterization of Sida Acuta Burm. f. Extracts with Respect to Its Anti-Cancer, Antioxidant and Enzyme Inhibitory Effects. Process Biochem. 2021, 105, 91–101. [Google Scholar] [CrossRef]
- Liu, K.-G.; Bigdeli, F.; Sharifzadeh, Z.; Gholizadeh, S.; Morsali, A. Role of Metal-Organic Framework Composites in Removal of Inorganic Toxic Contaminants. J. Clean. Prod. 2023, 404, 136709. [Google Scholar] [CrossRef]
- Niu, B.; Chen, Y.; Zhang, L.; Tan, J. Organic-Inorganic Hybrid Nanomaterials Prepared via Polymerization-Induced Self-Assembly: Recent Developments and Future Opportunities. Polym. Chem. 2022, 2554–2569. [Google Scholar] [CrossRef]
- Nandana, C.N.; Christeena, M.; Bharathi, D. Synthesis and Characterization of Chitosan/Silver Nanocomposite Using Rutin for Antibacterial, Antioxidant and Photocatalytic Applications. J. Clust. Sci. 2022, 33, 269–279. [Google Scholar] [CrossRef]
- Ramachandran, G.; Alharbi, N.S.; Chackaravarthy, G.; Kanisha Chelliah, C.; Rajivgandhi, G.; Maruthupandy, M.; Quero, F.; Natesan, M.; Li, W.J. Chitosan/Silver Nanocomposites Enhanced the Biofilm Eradication in Biofilm Forming Gram Positive S. Aureus. J. King Saud Univ.-Sci. 2023, 35, 102597. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Vatankhah, M.; Hassanisaadi, M.; Kennedy, J.F. Chitosan-Based Nanocomposites as Coatings and Packaging Materials for the Postharvest Improvement of Agricultural Product: A Review. Carbohydr. Polym. 2023, 309, 120666. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Dong, Q.; Xu, C.; Deng, S.; Kang, Y.; Fan, M.; Li, L. Application of Functionalized Chitosan in Food: A Review. Int. J. Biol. Macromol. 2023, 235. [Google Scholar] [CrossRef] [PubMed]
- Wongpreecha, J.; Polpanich, D.; Suteewong, T.; Kaewsaneha, C.; Tangboriboonrat, P. One-Pot, Large-Scale Green Synthesis of Silver Nanoparticles-Chitosan with Enhanced Antibacterial Activity and Low Cytotoxicity. Carbohydr. Polym. 2018, 199, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; MubarakAli, D.; Vadivelu, J.; Manjunath Kamath, S.; Syed, A.; Elgorban, A.M. Synthesis of Biocompatible Chitosan Decorated Silver Nanoparticles Biocomposites for Enhanced Antimicrobial and Anticancer Property. Process Biochem. 2020, 99, 348–356. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Bharathi, D.; Saravanan, M.; Manikandan, E.; Kumar, S.S.; Pugazhendhi, A. Biogenesis of Copper Oxide Nanoparticles (CuONPs) Using Sida Acuta and Their Incorporation over Cotton Fabrics to Prevent the Pathogenicity of Gram Negative and Gram Positive Bacteria. J. Photochem. Photobiol. B Biol. 2018. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Houreld, N.N.; Kroukamp, E.M.; Abrahamse, H. Cellular Imaging and Bactericidal Mechanism of Green-Synthesized Silver Nanoparticles against Human Pathogenic Bacteria. J. Photochem. Photobiol. B Biol. 2018, 178, 259–269. [Google Scholar] [CrossRef]
- Sathiskumar, S.; Vanaraj, S.; Sabarinathan, D.; Preethi, K. Evaluation of Antibacterial and Antibiofilm Activity of Synthesized Zinc-Hydroxyapatite Biocomposites from Labeo Rohita Fish Scale Waste. Mater. Res. Express 2018, 5, 025407. [Google Scholar] [CrossRef]
- Bharathi, D.; Preethi, S.; Abarna, K.; Nithyasri, M.; Kishore, P.; Deepika, K. Bio-Inspired Synthesis of Flower Shaped Iron Oxide Nanoparticles (FeONPs) Using Phytochemicals of Solanum Lycopersicum Leaf Extract for Biomedical Applications. Biocatal. Agric. Biotechnol. 2020, 27, 101698. [Google Scholar] [CrossRef]
- George, I.E.; Cherian, T.; Ragavendran, C.; Mohanraju, R.; Dailah, H.G.; Hassani, R.; Alhazmi, H.A.; Khalid, A.; Mohan, S. One-Pot Green Synthesis of Silver Nanoparticles Using Brittle Star Ophiocoma Scolopendrina: Assessing Biological Potentialities of Antibacterial, Antioxidant, Anti-Diabetic and Catalytic Degradation of Organic Dyes. Heliyon 2023, 9, e14538. [Google Scholar] [CrossRef]
- Thomas, T.; Thalla, A.K. Synthesis of Silver Nanoparticles Using Myristica Fragrans Seed Shell: Assessment of Antibacterial, Antioxidant Properties and Photocatalytic Degradation of Dyes. J. Environ. Chem. Eng. 2023, 11, 109585. [Google Scholar] [CrossRef]
- Vankudoth, S.; Dharavath, S.; Veera, S.; Maduru, N.; Chada, R.; Chirumamilla, P.; Gopu, C.; Taduri, S. Green Synthesis, Characterization, Photoluminescence and Biological Studies of Silver Nanoparticles from the Leaf Extract of Muntingia Calabura. Biochem. Biophys. Res. Commun. 2022, 630, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Alshameri, A.W.; Owais, M.; Altaf, I.; Farheen, S. Rumex Nervosus Mediated Green Synthesis of Silver Nanoparticles and Evaluation of Its in Vitro Antibacterial, and Cytotoxic Activity. OpenNano 2022, 8, 100084. [Google Scholar] [CrossRef]
- Soto-Robles, C.A.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Nava, O.; Vilchis-Nestor, A.R.; Lugo-Medina, E.; Ranjithkumar, R.; Castro-Beltrán, A. Study on the Effect of the Concentration of Hibiscus Sabdariffa Extract on the Green Synthesis of ZnO Nanoparticles. Results Phys. 2019, 15. [Google Scholar] [CrossRef]
- Luque, P.A.; Soto-Robles, C.A.; Nava, O.; Gomez-Gutierrez, C.M.; Castro-Beltran, A.; Garrafa-Galvez, H.E.; Vilchis-Nestor, A.R.; Olivas, A. Green Synthesis of Zinc Oxide Nanoparticles Using Citrus Sinensis Extract. J. Mater. Sci. Mater. Electron. 2018, 29, 9764–9770. [Google Scholar] [CrossRef]
- Laib, I.; Djahra Ali, B.; Boudebia, O. Green Synthesis of Silver Nanoparticles Using Helianthemum Lippii Extracts (Hl-NPs): Characterization, Antioxidant and Antibacterial Activities, and Study of Interaction with DNA. J. Organomet. Chem. 2023, 986, 122619. [Google Scholar] [CrossRef]
- Sukumar, D.T.; Gunasangkaran, G.; Arumugam, V.A.; Muthukrishnan, S. Effects of Biogenic Synthesis of Chitosan Entrapped Silver Nanoparticle from Aegle Marmelos on Human Cervical Cancer Cells (HeLa). J. Drug Deliv. Sci. Technol. 2022, 70, 103189. [Google Scholar] [CrossRef]
- Rezazadeh, N.H.; Buazar, F.; Matroodi, S. Synergistic Effects of Combinatorial Chitosan and Polyphenol Biomolecules on Enhanced Antibacterial Activity of Biofunctionalaized Silver Nanoparticles. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Liu, C.; Ling, J.; Yang, L.Y.; Ouyang, X.K.; Wang, N. Chitosan-Based Carbon Nitride-Polydopamine-silver Composite Dressing with Antibacterial Properties for Wound Healing. Carbohydr. Polym. 2023, 303, 120436. [Google Scholar] [CrossRef]
- Kocadag Kocazorbaz, E.; Moulahoum, H.; Tut, E.; Sarac, A.; Tok, K.; Yalcin, H.T.; Zihnioglu, F. Kermes Oak (Quercus Coccifera L.) Extract for a Biogenic and Eco-Benign Synthesis of Silver Nanoparticles with Efficient Biological Activities. Environ. Technol. Innov. 2021, 24, 102067. [Google Scholar] [CrossRef]
- Gecer, E.N.; Erenler, R. Biogenic Synthesis of Silver Nanoparticles Using Echium Vulgare: Characterisation, Quantitative Analysis of Bioactive Compounds, Antioxidant Activity and Catalytic Degradation. J. Indian Chem. Soc. 2023, 100, 101003. [Google Scholar] [CrossRef]
- Lunkov, A.; Shagdarova, B.; Konovalova, M.; Zhuikova, Y.; Drozd, N.; Il’ina, A.; Varlamov, V. Synthesis of Silver Nanoparticles Using Gallic Acid-Conjugated Chitosan Derivatives. Carbohydr. Polym. 2020, 234, 115916. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. Fun. Chitosan. 2020, 457–489. [Google Scholar] [CrossRef]
- Shahid-ul-Islam; Butola, B.S.; Verma, D. Facile Synthesis of Chitosan-Silver Nanoparticles onto Linen for Antibacterial Activity and Free-Radical Scavenging Textiles. Int. J. Biol. Macromol. 2019, 133, 1134–1141. [Google Scholar] [CrossRef]
- Choudhary, P.; Parandhaman, T.; Ramalingam, B.; Duraipandy, N.; Kiran, M.S.; Das, S.K. Fabrication of Nontoxic Reduced Graphene Oxide Protein Nanoframework as Sustained Antimicrobial Coating for Biomedical Application. ACS Appl. Mater. Interfaces 2017, 9, 38255–38269. [Google Scholar] [CrossRef]
- Singh, A.; Dubey, A.K. Various Biomaterials and Techniques for Improving Antibacterial Response. ACS Appl. Bio Mater. 2018, 1, 3–20. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green Synthesis of Silver Nanoparticles: Biomolecule-Nanoparticle Organizations Targeting Antimicrobial Activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic Synthesis of Silver Nanoparticles and Their Synergistic Effect with Antibiotics: A Study against Gram-Positive and Gram-Negative Bacteria. Nanomedicine Nanotechnology, Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Priya, K.; Vijayakumar, M.; Janani, B. Chitosan-Mediated Synthesis of Biogenic Silver Nanoparticles (AgNPs), Nanoparticle Characterisation and in Vitro Assessment of Anticancer Activity in Human Hepatocellular Carcinoma HepG2 Cells. Int. J. Biol. Macromol. 2020, 149, 844–852. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Tabriz Univ. Med. Sci. 2019, 7, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Aswini, R.; Murugesan, S.; Kannan, K. Bio-Engineered TiO2 Nanoparticles Using Ledebouria Revoluta Extract: Larvicidal, Histopathological, Antibacterial and Anticancer Activity. Int. J. Environ. Anal. Chem. 2021, 101, 2926–2936. [Google Scholar] [CrossRef]
- Kannan, K.; Radhika, D.; Vijayalakshmi, S.; Sadasivuni, K.K.; Ojiaku, A.A.; Verma, U. Facile Fabrication of CuO Nanoparticles via Microwave-Assisted Method: Photocatalytic, Antimicrobial and Anticancer Enhancing Performance. Int. J. Environ. Anal. Chem. 2022, 102, 1095–1108. [Google Scholar] [CrossRef]
- Bharathi, D.; Ranjithkumar, R.; Chandarshekar, B.; Bhuvaneshwari, V. Bio-Inspired Synthesis of Chitosan/Copper Oxide Nanocomposite Using Rutin and Their Anti-Proliferative Activity in Human Lung Cancer Cells. Int. J. Biol. Macromol. 2019, 141, 476–483. [Google Scholar] [CrossRef] [PubMed]







| Elements | Weight(s) |
|---|---|
| Ag | 53 |
| C | 02 |
| N | 14 |
| O | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharathi, D.; Thiruvengadam Nandagopal, J.G.; Lee, J.; Ranjithkumar, R. Facile Synthesis and Characterization of Chitosan Functionalized Silver Nanoparticles for Antibacterial and Anti-Lung Cancer Applications. Polymers 2023, 15, 2700. https://doi.org/10.3390/polym15122700
Bharathi D, Thiruvengadam Nandagopal JG, Lee J, Ranjithkumar R. Facile Synthesis and Characterization of Chitosan Functionalized Silver Nanoparticles for Antibacterial and Anti-Lung Cancer Applications. Polymers. 2023; 15(12):2700. https://doi.org/10.3390/polym15122700
Chicago/Turabian StyleBharathi, Devaraj, Jaya Ganesh Thiruvengadam Nandagopal, Jintae Lee, and Rajamani Ranjithkumar. 2023. "Facile Synthesis and Characterization of Chitosan Functionalized Silver Nanoparticles for Antibacterial and Anti-Lung Cancer Applications" Polymers 15, no. 12: 2700. https://doi.org/10.3390/polym15122700