Functional Materials Made by Combining Hydrogels (Cross-Linked Polyacrylamides) and Conducting Polymers (Polyanilines)—A Critical Review
Abstract
:1. Introduction
2. Combination of Polyacrylamides and Polyanilines
2.1. Synthesis
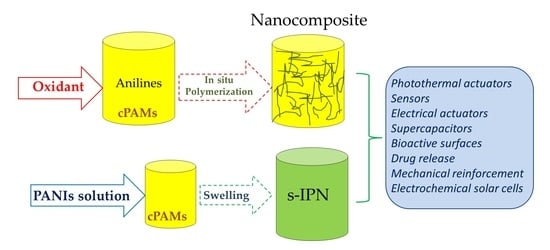
2.1.1. Nanocomposites
- −
- Hydrogel (cPAM) formation around nanoparticles of PANIs
2.1.2. Formation of a Semi-Interpenetrated Network (s-IPN)
- −
- Loading cPAM with PANIs from solution.
2.1.3. Grafted PANI Chains (gPC)
3. Technological Applications
3.1. Photothermal Actuators
3.1.1. Light
3.1.2. Microwaves and Radiofrequency
3.2. Sensors
- (i)
- Electrochemical sensors: As can be seen, the conductive matrix can be connected to a base electrode. The large surface area of the conductive network can function as an electrode or the PANIs can be used to connect redox enzymes which are immobilized inside the cPAM matrix.
- (ii)
- Electromechanical sensors: By compression of the combined material, the resistance decreases. In this way, pressure can be measured by monitoring the resistance.
- (iii)
- Conductivity sensors: The extended PANIs network exposes a large surface to the hydrogel. Upon absorption of an analyte (volatiles, molecules, ions) into the gel matrix, the analyte interacts with the PANIs, changing its electronic properties (e.g., conductivity).
3.2.1. Electrochemical
3.2.2. Electromechanical
3.2.3. Conductivity
3.3. Electrical Actuators
3.4. Supercapacitors
3.5. Bioactive Surfaces
3.6. Drug Release
3.7. Mechanical Reinforcement
3.8. Electrochemical Solar Cells
4. Conclusions and Future Outlook
Funding
Conflicts of Interest
Abbreviations
| AA | acrylic acid |
| AAm | Acrylamide |
| AC/DC | alternating current/direct current |
| AMPS | Acryamidopropanesulfonic acid |
| APS | ammonium persulfate |
| BIS | N,N’-Methylenebisacrylamide |
| cPAM | cross-linked polyacrylamides |
| CV | cyclic voltammetry |
| DMA | dynamic mechanical analysis |
| DMSO | dimethylsulfoxide |
| dPG | dendritic polyglycerol |
| DPV | differential pulse voltammetry |
| DSSC | dye-sensitized solar cell |
| EG | Ethylene glycol |
| GF | gauge factor |
| GO | Graphene oxide |
| HEMA | hydroxyethylmethacrylate |
| HPA | hydroxyapatite |
| IA | itaconic acid |
| IPN | interpenetrated network |
| ISP | in situ polymerization |
| LCST | lower critical solution temperature |
| LOD | lowest detection limit |
| MEO2MA | methoxyethoxy) ethyl methacrylate |
| MWNT-COOH | multiwall carbon nanotubes (carboxylated) |
| NC | nanocomposite |
| NIPAM | N-isopropylacrylamide |
| NIR | near infrared |
| NMP | N-methylpyrrolidone |
| NP | nanoparticles |
| OEGMA | oligo(ethylene glycol) methacrylate |
| PANI | Polyaniline |
| PANI(EB) | polyaniline in its deprotonated state (emeraldine base) |
| PANI(ES) | polyaniline in its protonated state (emeraldine salt) |
| PNMANI | poly(N-methylaniline) |
| PPy | polypyrrole |
| PTA | photothermal actuator |
| PVA | polyvinylalcohol |
| rGO | reduced graphene oxide |
| RT | room temperature |
| SEM | scanning electron microscopy |
| s-IPN | semi-interpenetrated network |
| SPAN | sulfonated polyanilline |
| TEMED | N, N, N’, N-tetramethylethylenediamine |
| UCST | upper critical solution temperature |
| UV | ultraviolet |
| αCD | α-cyclodextrin |
References
- Zhang, H.-P.; Cao, J.-J.; Jiang, W.-B.; Yang, Y.-Q.; Zhu, B.-Y.; Liu, X.-Y.; Wu, Y.; Sun, X.; Essouma, A.F.B.E.; Liu, J.; et al. Synthesis and Mechanical Properties of Polyacrylamide Gel Doped with Graphene Oxide. Energies 2022, 15, 5714. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Polyacrylamide Gel Electrophoresis. Cold Spring Harb. Protoc. 2020, 12, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Alsubaie, F.M.; Alothman, O.Y.; Alshammari, B.A.; Fouad, H. Facile Synthesis of Hydrophilic Homo-Polyacrylamides via Cu(0)-Mediated Reversible Deactivation Radical Polymerization. Polymers 2021, 13, 1947. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, S.; Zhang, Y.; Yin, H.; Feng, Y. Hydrophobically Associating Polyacrylamide “Water-in-Water” Emulsion Prepared by Aqueous Dispersion Polymerization: Synthesis, Characterization and Rheological Behavior. Molecules 2023, 28, 2698. [Google Scholar] [CrossRef] [PubMed]
- Barbero, C.A.; Martínez, M.V.; Acevedo, D.F.; Molina, M.A.; Rivarola, C.R. Cross-Linked Polymeric Gels and Nanocomposites: New Materials and Phenomena Enabling Technological Applications. Macromol 2022, 2, 440–475. [Google Scholar] [CrossRef]
- Qi, X.; Liu, J.; Wang, C.; Li, S.; Li, X.; Liang, Y.; Sarfaraz, K. Synthesis of the Hydrophobic Cationic Polyacrylamide (PADD) Initiated by Ultrasonic and its Flocculation and Treatment of Coal Mine Wastewater. Processes 2020, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.; Zhao, J.; Sun, C. Adsorption of Anionic Polyacrylamide onto Coal and Kaolinite Calculated from the Extended DLVO Theory Using the van Oss-Chaudhury-Good Theory. Polymers 2018, 10, 113. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues Sousa, H.; Lima, I.S.; Neris, L.M.L.; Silva, A.S.; Santos Nascimento, A.M.S.; Araújo, F.P.; Ratke, R.F.; Silva, D.A.; Osajima, J.A.; Bezerra, L.R.; et al. Superabsorbent Hydrogels Based to Polyacrylamide/Cashew Tree Gum for the Controlled Release of Water and Plant Nutrients. Molecules 2021, 26, 2680. [Google Scholar] [CrossRef]
- Komarova, G.A.; Kozhunova, E.Y.; Potemkin, I.I. Behavior of PNIPAM Microgels in Different Organic Solvents. Molecules 2022, 27, 8549. [Google Scholar] [CrossRef]
- Grigorova, K.; Kostova, B.; Georgieva, D.; Apostolov, A.; Vassileva, E. Polyacrylamide/poly(2-(dimethylamino) Ethyl Methacrylate) Interpenetrating Polymer Networks as Drug Delivery Systems for Diclofenac Sodium. Gels 2022, 8, 780. [Google Scholar] [CrossRef]
- Bi, Y.-Z.; Wen, J.-M.; Wu, H.-L.; Du, Y.-J. Evaluation of Performance of Polyacrylamide-Modified Compacted Clay as a Gas Barrier: Water Retention and Gas Permeability and Diffusion Characteristics. Appl. Sci. 2022, 12, 8379. [Google Scholar] [CrossRef]
- Hamri, S.; Bouchaour, T.; Lerari, D.; Bouberka, Z.; Supiot, P.; Maschke, U. Cleaning of Wastewater Using Crosslinked Poly(Acrylamide-co-Acrylic Acid) Hydrogels: Analysis of Rotatable Bonds, Binding Energy and Hydrogen Bonding. Gels 2022, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Safronov, A.P.; Mikhnevich, E.A.; Lotfollahi, Z.; Blyakhman, F.A.; Sklyar, T.F.; Larrañaga Varga, A.; Medvedev, A.I.; Fernández Armas, S.; Kurlyandskaya, G.V. Polyacrylamide Ferrogels with Magnetite or Strontium Hexaferrite: Next Step in the Development of Soft Biomimetic Matter for Biosensor Applications. Sensors 2018, 18, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santhamoorthy, M.; Vy Phan, T.T.; Ramkumar, V.; Raorane, C.J.; Thirupathi, K.; Kim, S.-C. Thermo-Sensitive Poly (N-isopropylacrylamide-co-polyacrylamide) Hydrogel for pH-Responsive Therapeutic Delivery. Polymers 2022, 14, 4128. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, K.; Phan, T.T.V.; Santhamoorthy, M.; Ramkumar, V.; Kim, S.-C. pH and Thermoresponsive PNIPAm-co-Polyacrylamide Hydrogel for Dual Stimuli-Responsive Controlled Drug Delivery. Polymers 2023, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Díaz Lantada, A.; Mazarío Picazo, N.; Guttmann, M.; Wissmann, M.; Schneider, M.; Worgull, M.; Hengsbach, S.; Rupp, F.; Bade, K.; Plaza, G.R. Soft-Lithography of Polyacrylamide Hydrogels Using Microstructured Templates: Towards Controlled Cell Populations on Biointerfaces. Materials 2020, 13, 1586. [Google Scholar] [CrossRef] [Green Version]
- Ambreen, J.; Haleem, A.; Shah, A.A.; Mushtaq, F.; Siddiq, M.; Bhatti, M.A.; Shah Bukhari, S.N.U.; Chandio, A.D.; Mahdi, W.A.; Alshehri, S. Facile Synthesis and Fabrication of NIPAM-Based Cryogels for Environmental Remediation. Gels 2023, 9, 64. [Google Scholar] [CrossRef]
- Shchapova, E.; Titov, E.; Gurkov, A.; Nazarova, A.; Borvinskaya, E.; Timofeyev, M. Durability of Implanted Low-Density Polyacrylamide Hydrogel Used as a Scaffold for Microencapsulated Molecular Probes inside Small Fish. Polymers 2022, 14, 3956. [Google Scholar] [CrossRef]
- Cennamo, N.; Arcadio, F.; Capasso, F.; Maniglio, D.; Zeni, L.; Bossi, A.M. Non-Specific Responsive Nanogels and Plasmonics to Design MathMaterial Sensing Interfaces: The Case of a Solvent Sensor. Sensors 2022, 22, 10006. [Google Scholar] [CrossRef]
- Molina, M.A.; Rivarola, C.R.; Broglia, M.F.; Acevedo, D.F.; Barbero, C.A. Smart surfaces: Reversible switching of a polymeric hydrogel topography. Soft Matter 2012, 8, 307–310. [Google Scholar] [CrossRef]
- Rivarola, C.R.; Biasutti, M.A.; Barbero, C.A. A visible light photoinitiator system to produce acrylamide based smart hydrogels: Ru(bpy)3+2 as photopolymerization initiator and molecular probe of hydrogel microenvironments. Polymer 2009, 50, 3145–3152. [Google Scholar] [CrossRef]
- Sorkhabi, T.S.; Samberan, M.F.; Ostrowski, K.A.; Zajdel, P.; Stempkowska, A.; Gawenda, T. Electrospinning of Poly (Acrylamide), Poly (Acrylic Acid) and Poly (Vinyl Alcohol) Nanofibers: Characterization and Optimization Study on the Effect of Different Parameters on Mean Diameter Using Taguchi Design of Experiment Method. Materials 2022, 15, 5876. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Aggas, J.R.; Abasi, S.; Smith, B.; Zimmerman, M.; Deprest, M.; Guiseppi-Elie, A. Microfabricated and 3-D printed soft bioelectronic constructs from PAn-PAAMPSA-containing hydrogels. Bioengineering 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Rangel-Olivares, F.R.; Arce-Estrada, E.M.; Cabrera-Sierra, R. Synthesis and Characterization of Polyaniline-Based Polymer Nanocomposites as Anti-Corrosion Coatings. Coatings 2021, 11, 653. [Google Scholar] [CrossRef]
- Firda, P.B.D.; Malik, Y.T.; Oh, J.K.; Wujcik, E.K.; Jeon, J.-W. Enhanced Chemical and Electrochemical Stability of Polyaniline-Based Layer-by-Layer Films. Polymers 2021, 13, 2992. [Google Scholar] [CrossRef]
- Mocioiu, A.-M.; Tudor, I.A.; Mocioiu, O.C. Application of Polyaniline for Flexible Semiconductors. Coatings 2021, 11, 49. [Google Scholar] [CrossRef]
- Fungaro, D.A. Sulfonated Polyaniline Coated Mercury Film Electrodes for Voltammetric Analysis of Metals in Water. Sensors 2001, 1, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Direksilp, C.; Sirivat, A. Synthesis and Characterization of Hollow-Sphered Poly(N-methyaniline) for Enhanced Electrical Conductivity Based on the Anionic Surfactant Templates and Doping. Polymers 2020, 12, 1023. [Google Scholar] [CrossRef]
- Butoi, B.; Groza, A.; Dinca, P.; Balan, A.; Barna, V. Morphological and Structural Analysis of Polyaniline and Poly(o-anisidine) Layers Generated in a DC Glow Discharge Plasma by Using an Oblique Angle Electrode Deposition Configuration. Polymers 2017, 9, 732. [Google Scholar] [CrossRef] [Green Version]
- Barbero, C.; Kötz, R. Electrochemical formation of a self-doped conductive polymer in the absence of a supporting electrolyte. The copolymerization of o-aminobenzenesulfonic acid and aniline. Adv. Mater. 1994, 6, 577–580. [Google Scholar] [CrossRef]
- Abel, S.B.; Frontera, E.; Acevedo, D.; Barbero, C.A. Functionalization of Conductive Polymers through Covalent Postmodification. Polymers 2023, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- Barbero, C.; Miras, M.C.; Schnyder, B.; Haas, O.; Kötz, R. Sulfonated polyaniline films as cation insertion electrodes for battery applications Part 1. Structural and electrochemical characterization. J. Mater. Chem 1994, 4, 1775–1783. [Google Scholar] [CrossRef]
- Cao, G.; Xu, J.; Cai, S.; Chen, Y.; Zhou, D.; Zhang, H.; Jiang, C.; Zhang, G.; Tian, Y. Highly Conductive and Dispersible Polyaniline Microtubes Controlled by Methyl Orange. ACS Appl. Polym. Mater. 2023, 5, 593–601. [Google Scholar] [CrossRef]
- Huang, W.S.; MacDiarmid, A.G. Optical properties of polyaniline. Polymer 1993, 34, 1833–1845. [Google Scholar] [CrossRef]
- Tsao, C.-W.; Chang, C.-Y.; Chien, P.-Y. Microwave-Assisted Solvent Bonding for Polymethyl Methacrylate Microfluidic Device. Micromachines 2022, 13, 1131. [Google Scholar] [CrossRef]
- Bongiovanni Abel, S.; Molina, M.; Rivarola, C.R.; Barbero, C.A. Pickering emulsions stabilized with PANI-NP. Study of the thermoresponsive behavior under heating and radiofrequency irradiation. J. Appl. Polym. Sci. 2021, 138, 50625. [Google Scholar] [CrossRef]
- Parvez, M.S.; Rahman, M.M.; Samykano, M.; Yeakub Ali, M. Electrochemical characterization and joule heating performance of polyaniline incorporated cotton fabric. Phys. Chem. Earth 2023, 129, 103323. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Pyarasani, R.D.; Jayaramudu, T.; John, A. Polyaniline-based conducting hydrogels. J. Mater. Sci. 2019, 54, 974–996. [Google Scholar] [CrossRef]
- Riaz, U.; Singh, N.; Rashnas Srambikal, F.; Fatima, S. A review on synthesis and applications of polyaniline and polypyrrole hydrogels. Polym. Bull. 2023, 80, 1085–1116. [Google Scholar] [CrossRef]
- Mir, A.; Kumar, A.; Riaz, U. A short review on the synthesis and advance applications of polyaniline hydrogels. RSC Adv. 2022, 12, 19122–19132. [Google Scholar] [CrossRef] [PubMed]
- Guiseppi-Elie, A. Electroconductive hydrogels: Synthesis, characterization and biomedical applications. Biomaterials 2010, 31, 2701–2716. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.W.; Portillo Lara, R.; Mogadam, E.; Hsiang Yu, C.; Kimball, W.; Annabi, N. Rational design of microfabricated electroconductive hydrogels for biomedical applications. Prog. Polym. Sci. 2019, 92, 135–157. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Tang, Q.; Hu, D.; Sun, X.; Li, Q.; Wu, J. Electric field sensitivity of conducting hydrogels with interpenetrating polymer network structure. Colloids Surf. A Physicochem. Eng. Asp. 2009, 346, 177–183. [Google Scholar] [CrossRef]
- González, F.; Tiemblo, P.; Hoyos, M. In-Situ Approaches for the Preparation of Polythiophene-Derivative Cellulose Composites with High Flexibility and Conductivity. Appl. Sci. 2019, 9, 3371. [Google Scholar] [CrossRef] [Green Version]
- Taşdelen, B. Preparation and characterization of conducting hydrogels composite made of polyaniline, polyacrylamide and kaolin. Mater. Today 2018, 5, 15983–15989. [Google Scholar] [CrossRef]
- Okutan, M.; Yavuz, E.; Ahlatcıoğlu Özerol, E.; Şenkal, B.F.; Yalçın, O.; Yıldız, A. Impedance spectroscopy of polyaniline coated hydrogel. Polym. Bull. 2021, 78, 4473–4486. [Google Scholar] [CrossRef]
- Qian, C.; Li, Y.; Chen, C.; Han, L.; Han, Q.; Liu, L.; Lu, Z. A stretchable and conductive design based on multi-responsive hydrogel for self-sensing actuators. Chem. Eng. J. 2023, 454, 140263. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Zhao, X.; Du, T.; Zhu, J.; Xie, Y.; Wu, F.; Wang, Y.; Guan, M. Poly(N-Isopropylacrylamide) Based Electrically Conductive Hydrogels and Their Applications. Gels 2022, 8, 280. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S. In situ preparation of polyaniline within neutral, anionic, and cationic superporous cryogel networks as conductive, semi-interpenetrating polymer network cryogel composite systems. J. Appl. Polym. Sci. 2016, 133, 44137. [Google Scholar] [CrossRef]
- Mulko, L.E.; Cuello, E.A.; Barbero, C.A.; Pino, G.A.; Molina, M.; Rossa, M. Remote radiofrequency triggering of topography changes in a surface micropatterned PANI@PNIPAM nanocomposite. Appl. Surf. Sci. 2020, 509, 145370. [Google Scholar] [CrossRef]
- Azam, F.; Ahmad, F.; Ahmad, S.; Zafar, M.S.; Ulker, Z. Preparation and Characterization of Alginate Hydrogel Fibers Reinforced by Cotton for Biomedical Applications. Polymers 2022, 14, 4707. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Rashid, M.; Rahman, M.M.; Miah, M.A.J.; Tauer, K.; Gafur, M.A. Surface modification of temperature-responsive polymer particles by an electrically conducting polyaniline shell layer. Polym. Int. 2014, 63, 667–673. [Google Scholar] [CrossRef]
- Gospodinova, N.; Terlemezyan, L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Prog. Polym. Sci. 1998, 23, 1443–1484. [Google Scholar] [CrossRef]
- Holze, R. Overoxidation of Intrinsically Conducting Polymers. Polymers 2022, 14, 1584. [Google Scholar] [CrossRef]
- Tang, S.-J.; Wang, A.-T.; Lin, S.-Y.; Huang, K.-Y.; Yang, C.-C.; Yeh, J.-M.; Chiu, K.-C. Polymerization of aniline under various concentrations of APS and HCl. Polym. J. 2011, 43, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Shen, H.; Lan, J.; Wu, H.; Wang, L.; Zhou, J. Dual-network polyacrylamide/carboxymethyl chitosan-grafted-polyaniline conductive hydrogels for wearable strain sensors. Carbohydr. Polym. 2022, 295, 119848. [Google Scholar] [CrossRef]
- Kaith, B.S.; Sharma, K.; Kumar, V.; Kalia, S.; Swart, H.C. Fabrication and characterization of gum ghatti-polymethacrylic acid based electrically conductive hydrogels. Synth. Met. 2014, 187, 61–67. [Google Scholar] [CrossRef]
- Lu, C.-H.; Guo, W.; Qi, X.-J.; Neubauer, A.; Paltiel, Y.; Willner, I. Hemin-G-quadruplex-crosslinked poly-N-isopropylacrylamide hydrogel: A catalytic matrix for the deposition of conductive polyaniline. Chem. Sci. 2015, 6, 6659–6664. [Google Scholar] [CrossRef] [Green Version]
- Molina, M.A.; Rivarola, C.R.; Barbero, C.A. Effect of copolymerization and semi-interpenetration with conducting polyanilines on the physicochemical properties of poly(N-isopropylacrylamide) based thermosensitive hydrogels. Eur. Polym. J. 2011, 47, 1977–1984. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Bajpai, J.; Soni, S.N. Electroactive actuation and conductive behavior of polyaniline-impregnated blood compatible nanocomposites. J. Compos. Mater. 2011, 45, 485–497. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Bajpai, J.; Soni, S.N. Preparation and characterization of electrically conductive composites of poly(vinyl alcohol)-g-poly(acrylic acid) hydrogels impregnated with polyaniline (PANI). Express Polym. Lett. 2008, 2, 26–39. [Google Scholar] [CrossRef]
- Martínez, M.V.; Bongiovanni Abel, S.; Rivero, R.; Miras, M.C.; Rivarola, C.R.; Barbero, C.A. Polymeric nanocomposites made of a conductive polymer and a thermosensitive hydrogel: Strong effect of the preparation procedure on the properties. Polymer 2015, 78, 94–103. [Google Scholar] [CrossRef]
- Celik, M.U.; Ekici, S. Polyacrylamide-polyaniline composites: The effect of crosslinking on thermal, swelling, porosity, crystallinity, and conductivity properties. Colloid. Polym. Sci. 2019, 297, 1331–1343. [Google Scholar] [CrossRef]
- Taşdelen, B. Conducting hydrogels based on semi-interpenetrating networks of polyaniline in poly(acrylamide-co-itaconic acid) matrix: Synthesis and characterization. Polym. Adv. Technol. 2017, 28, 1865–1871. [Google Scholar] [CrossRef]
- Sharma, K.; Kaith, B.S.; Kumar, V.; Kumar, V.; Som, S.; Kalia, S.; Swart, H.C. Synthesis and properties of poly(acrylamide-aniline)-grafted gum ghatti based nanospikes. RSC Adv. 2013, 3, 25830–25839. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, H. Polyaniline nanofiber-reinforced conducting hydrogel with unique pH-sensitivity. Soft Matter 2011, 7, 9388–9393. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Sun, H.; Lin, J.; Fan, S.; Hu, D. Polyaniline/polyacrylamide conducting composite hydrogel with a porous structure. Carbohydr. Polym. 2008, 74, 215–219. [Google Scholar] [CrossRef]
- Boruah, M.; Kalita, A.; Pokhrel, B.; Dolui, S.K.; Boruah, R. Synthesis and characterization of pH responsive conductive composites of poly(acrylic acid-co-acrylamide) impregnated with polyaniline by interfacial polymerization. Adv. Polym. Technol. 2013, 32, E520–E530. [Google Scholar] [CrossRef]
- Dai, T.; Qing, X.; Wang, J.; Shen, C.; Lu, Y. Interfacial polymerization to high-quality polyacrylamide/polyaniline composite hydrogels. Compos. Sci. Technol. 2010, 70, 498–503. [Google Scholar] [CrossRef]
- Barbero, C.A. Diverse Methods to Nanomanufacture Colloidal Dispersions of Polyaniline without Templates. Nanomanufacturing 2023, 3, 57–90. [Google Scholar] [CrossRef]
- Prabhakar, R.; Kumar, D. Influence of Dopant Ions on the Properties of Conducting Polyacrylamide/Polyaniline Hydrogels. Polym. Plast. Technol. Eng. 2016, 55, 46–53. [Google Scholar] [CrossRef]
- Miranda, D.O.; Dorneles, M.F.; Oréfice, R.L. Core–shell electrospun fiber architecture used as a nanoreactor to synthesize conjugated polymers. Polym. Int. 2022, 72, 434–439. [Google Scholar] [CrossRef]
- Karbarz, M.; Gniadek, M.; Donten, M.; Stojek, Z. Intra-channel modification of environmentally sensitive poly(N-isopropylacrylamide) hydrogel with polyaniline using interphase synthesis. Electrochem. Commun 2011, 13, 714–718. [Google Scholar] [CrossRef]
- Mao, J.; Yu, Q.J.; Wang, S. Preparation of multifunctional hydrogels with pore channels using agarose sacrificial templates and its applications. Polym. Adv. Technol. 2021, 32, 1752–1762. [Google Scholar] [CrossRef]
- Abel, S.B.; Rivarola, C.R.; Barbero, C.A.; Molina, M. Electromagnetic radiation driving of volume changes in nanocomposites made of a thermosensitive hydrogel polymerized around conducting polymer nanoparticles. RSC Adv. 2020, 10, 9155–9164. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z. Nanoparticle–Hydrogel Based Sensors: Synthesis and Applications. Catalysts 2022, 12, 1096. [Google Scholar] [CrossRef]
- Valade, D.; Wong, L.K.; Jeon, Y.; Jia, Z.; Monteiro, M.J. Polyacrylamide hydrogel membranes with controlled pore sizes. J. Polym. Sci. A Polym. Chem. 2013, 51, 129–138. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I. Polyaniline: Thin films and colloidal dispersions. Pure Appl. Chem. 2005, 77, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Molina, M.A.; Rivarola, C.R.; Miras, M.C.; Lescano, D.; Barbero, C.A. Nanocomposite synthesis by absorption of nanoparticles into macroporous hydrogels. Building a chemomechanical actuator driven by electromagnetic radiation. Nanotechnology 2011, 22, 245504. [Google Scholar] [CrossRef]
- Barbero, C.; Miras, M.C.; Kötz, R.; Haas, O. Sulphonated polyaniline (SPAN) films as cation insertion electrodes for battery applications part II: Exchange of mobile species in aqueous and non-aqueous solutions. J. Electroanal. Chem. 1997, 437, 191–198. [Google Scholar] [CrossRef]
- Tao, Y.; Zhao, J.X.; Wu, C.X. Polyacrylamide hydrogels with trapped sulfonated polyaniline. Eur. Polym. J. 2005, 41, 1342–1349. [Google Scholar] [CrossRef]
- Martinez, M.V.; Molina, M.A.; Abel, S.B.; Barbero, C.A. Large Swelling Capacities of Crosslinked Poly(N-isopropylacrylamide) Gels in Organic Solvents. MRS Adv. 2018, 3, 3735–3740. [Google Scholar] [CrossRef]
- Cao, Y.; Smith, P.; Heeger, A.J. Counter-ion induced processibility of conducting polyaniline and of conducting polyblends of polyaniline in bulk polymers. Synth. Met. 1992, 48, 91–97. [Google Scholar] [CrossRef]
- Monge, N.E.; Miras, M.C.; Barbero, C.A. High-throughput screening method to detect amphiphilic counterions able to solubilize conducting polymers. J. Comb. Chem. 2010, 12, 814–817. [Google Scholar] [CrossRef]
- Martinez, M.V.; Molina, M.; Barbero, C.A. Poly(N-isopropylacrylamide) Cross-Linked Gels as Intrinsic Amphiphilic Materials: Swelling Properties Used to Build Novel Interphases. J. Phys. Chem. B 2018, 122, 9038–9048. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-X.; Li, P.-Y.; Li, J.-M.; He, W.-D. Preparation and investigation of conductive hydrogels of polyacrylamide-g-polyaniline. Acta Polym. Sin. 2016, 11, 1563–1571. [Google Scholar]
- Bongiovanni Abel, S.; Riberi, K.; Rivarola, C.R.; Molina, M.; Barbero, C.A. Synthesis of a Smart Conductive Block Copolymer Responsive to Heat and Near Infrared Light. Polymers 2019, 11, 1744. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, M.A.; Sokolova, M.P.; Bobrova, N.V.; Kasatkin, I.A.; Lahderanta, E.; Elyashevich, G.K. Capacitance properties and structure of electroconducting hydrogels based on copoly(aniline–p-phenylenediamine) and polyacrylamide. J. Power Sources 2016, 304, 102–110. [Google Scholar] [CrossRef]
- Pang, Q.; Wu, K.; Jiang, Z.; Shi, Z.; Si, Z.; Wang, Q.; Cao, Y.; Hou, R.; Zhu, Y. A Polyaniline Nanoparticles Crosslinked Hydrogel with Excellent Photothermal Antibacterial and Mechanical Properties for Wound Dressing. Macromol. Biosci. 2022, 22, 2100386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lo, C.-Y.; Ruan, L.; Pi, C.-H.; Kim, C.; Alsaid, Y.; Frenkel, I.; Rico, R.; Tsao, T.-C.; He, X. Somatosensory actuator based on stretchable conductive photothermally responsive hydrogel. Sci. Robot. 2021, 6, eabd5483. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Guo, Y.; Ma, P.X.; Guo, B. Rapid thermal responsive conductive hybrid cryogels with shape memory properties, photothermal properties and pressure dependent conductivity. J. Colloid Interface Sci. 2018, 526, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.; Wedepohl, S.; Calderón, M. Polymeric near-infrared absorbing dendritic nanogels for efficient in vivo photothermal cancer therapy. Nanoscale 2016, 8, 5852–5856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depa, K.; Strachota, A.; Šlouf, M.; Brus, J.; Cimrová, V. Synthesis of conductive doubly filled poly(N-isopropylacrylamide)-polyaniline-SiO2 hydrogels. Sens. Actuators B Chem. 2017, 244, 616–634. [Google Scholar] [CrossRef]
- Rivero, R.E.; Molina, M.A.; Rivarola, C.R.; Barbero, C.A. Pressure and microwave sensors/actuators based on smart hydrogel/conductive polymer nanocomposite. Sens. Actuators B Chem. 2014, 190, 270–278. [Google Scholar] [CrossRef]
- Barbero, C.A.; Acevedo, D.F. Manufacturing Functional Polymer Surfaces by Direct Laser Interference Patterning (DLIP): A Polymer Science View. Nanomanufacturing 2022, 2, 229–264. [Google Scholar] [CrossRef]
- Hassan, M.H.; Vyas, C.; Grieve, B.; Bartolo, P. Recent Advances in Enzymatic and Non-Enzymatic Electrochemical Glucose Sensing. Sensors 2021, 21, 4672. [Google Scholar] [CrossRef]
- Gniadek, M.; Malinowska, S.; Kaniewska, K.; Karbarz, M.; Stojek, Z.; Donten, M. Construction of multifunctional materials by intrachannel modification of NIPA hydrogel with PANI-metal composites. J. Electroanal. Chem. 2018, 812, 273–281. [Google Scholar] [CrossRef]
- Das, J.; Sarkar, P. Enzymatic electrochemical biosensor for urea with a polyaniline grafted conducting hydrogel composite modified electrode. RSC Adv. 2016, 6, 92520–92533. [Google Scholar] [CrossRef]
- Li, Y.; Gong, Q.; Liu, X.; Xia, Z.; Yang, Y.; Chen, C.; Qian, C. Wide temperature-tolerant polyaniline/cellulose/polyacrylamide hydrogels for high-performance supercapacitors and motion sensors. Carbohydr. Polym. 2021, 267, 118207. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wei, P.; Chen, G.; Liu, H.; Mugaanire, I.T.; Hou, K.; Zhu, M. Heterogeneous structured tough conductive gel fibres for stable and high-performance wearable strain sensors. J. Mater. Chem. A 2021, 9, 12265–12275. [Google Scholar] [CrossRef]
- Qin, H.; Chen, Y.; Huang, J.; Wei, Q. Bacterial Cellulose Reinforced Polyaniline Electroconductive Hydrogel with Multiple Weak H-Bonds as Flexible and Sensitive Strain Sensor. Macromol. Mater. Eng. 2021, 306, 2100159. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Chen, W.; Li, Q.; Yan, B.; Jin, X.; Ma, A.; Liu, H.; Zhao, W. Dually Synergetic Network Hydrogels with Integrated Mechanical Stretchability, Thermal Responsiveness, and Electrical Conductivity for Strain Sensors and Temperature Alertors. ACS Appl. Mater. Interfaces 2018, 10, 14045–14054. [Google Scholar] [CrossRef]
- Da Silva, L.B.J.; Oréfice, R.L. Synthesis and electromechanical actuation of a temperature, pH, and electrically responsive hydrogel. J. Polym. Res. 2014, 21, 466. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Cong, Y.; Zhang, H.; Xu, T.; Nie, L.; Fu, J. Ultrastretchable Strain Sensors and Arrays with High Sensitivity and Linearity Based on Super Tough Conductive Hydrogels. Chem. Mater. 2018, 30, 8062–8069. [Google Scholar] [CrossRef]
- Cavallo, P.; Acevedo, D.F.; Fuertes, M.C.; Soler-Illia, G.J.A.A.; Barbero, C.A. Understanding the sensing mechanism of polyaniline resistive sensors. Effect of humidity on sensing of organic volatiles. Sens. Actuators B Chem. 2015, 210, 574–580. [Google Scholar] [CrossRef]
- Demirci, S.; Silan, C.; Sahiner, N. Graphene oxide embedded P(AAm)/PANI cryogel polymer composites for sensor application against pesticide, nitro compound, and organic dyes. J. Macromol. Sci. A 2019, 56, 994–1003. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, M.; He, Q.; Vokoun, D.; Yin, G.; Xu, X.; Lyu, P. Axial Motion Characterization of a Helical Ionic Polymer Metal Composite Actuator and Its Application in 3-DOF Micro-Parallel Platforms. Actuators 2021, 10, 248. [Google Scholar] [CrossRef]
- Deng, Z.; Yu, R.; Guo, B. Stimuli-responsive conductive hydrogels: Design, properties, and applications. Mater. Chem. Front. 2021, 5, 2092–2123. [Google Scholar] [CrossRef]
- Shi, H.; Dai, Z.; Sheng, X.; Xia, D.; Shao, P.; Yang, L.; Luo, X. Conducting polymer hydrogels as a sustainable platform for advanced energy, biomedical and environmental applications. Sci. Total Environ. 2021, 786, 147430. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.Z.; Han, W.J.; Choi, H.J. Polyaniline Coated Core-Shell Typed Stimuli-Responsive Microspheres and Their Electrorheology. Polymers 2018, 10, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Ma, C.; Peng, L.; Yu, G. Conductive “smart” hybrid hydrogels with PNIPAM and nanostructured conductive polymers. Adv. Funct. Mater. 2015, 25, 1219–1225. [Google Scholar] [CrossRef]
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.C.-K.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Ajji, A.; Heuzey, M.-C. Fast thermal responsive hydrogels consisting of electrospun fibers with highly tunable conductivity. Sens. Actuators A Phys. 2023, 349, 114016. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.; Shi, X.; Han, D.; Liang, F. A thermally responsive host-guest conductive hydrogel with self-healing properties. Mater. Chem. Front. 2018, 2, 2212–2219. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, S.J.; Kim, S.J. Volume behavior of interpenetrating polymer network hydrogels composed of polyacrylic acid-co-poly(vinyl sulfonic acid)/polyaniline as an actuator. Smart Mater. Struct. 2006, 15, 1882–1886. [Google Scholar] [CrossRef]
- Siddhanta, S.K.; Gangopadhyay, R. Conducting polymer gel: Formation of a novel semi-IPN from polyaniline and crosslinked poly(2-acrylamido-2-methyl propanesulphonicacid). Polymer 2005, 46, 2993–3000. [Google Scholar] [CrossRef]
- Hong, X.; Fu, J.; Liu, Y.; Li, S.; Wang, X.; Dong, W.; Yang, S. Recent Progress on Graphene/Polyaniline Composites for High-performance Supercapacitors. Materials 2019, 12, 1451. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Wu, X.; Du, T.; Wang, Y.; Liu, Z.; Yang, W.; Yang, M. Prestretching-Enhanced Conductive Hydrogel Electrode for Supercapacitors with High Areal Capacitance and Excellent Stretching Stability. Energy Technol. 2023, in press. [Google Scholar] [CrossRef]
- Wang, D.; Yang, F.; Wang, C.; Chu, F.; Nan, J.; Chen, R. In-situ polymerization of PANI on hydrogel electrolyte enabling all-in-one supercapacitors mechanically stable at low temperatures. Chem. Eng. J. 2023, 455, 140949. [Google Scholar] [CrossRef]
- Hao, G.-P.; Hippauf, F.; Oschatz, M.; Wisser, F.M.; Leifert, A.; Nickel, W.; Mohamed-Noriega, N.; Zheng, Z.; Kaskel, S. Stretchable and semitransparent conductive hybrid hydrogels for flexible supercapacitors. ACS Nano 2014, 8, 7138–7146. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Yang, W.; Li, H. Recent advances in polyaniline-based micro-supercapacitors. Mater. Horiz. 2023, 10, 670–697. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Shen, L.; Lu, Y.; Hu, C.; Liang, Z.; Long, L.; Ning, N.; Chen, J.; Guo, Y.; Yang, Z.; et al. Intrinsic Antibacterial and Conductive Hydrogels Based on the Distinct Bactericidal Effect of Polyaniline for Infected Chronic Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 52308–52320. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Sun, H.; Fan, S.; Hu, D.; Lin, J. Superabsorbent conducting hydrogel from poly(acrylamide-aniline) with thermo-sensitivity and release properties. Carbohydr. Polym. 2008, 73, 473–481. [Google Scholar] [CrossRef]
- Molina, M.A.; Rivarola, C.R.; Barbero, C.A. Study on partition and release of molecules in superabsorbent thermosensitive nanocomposites. Polymer 2012, 53, 445–453. [Google Scholar] [CrossRef]
- Planes, G.A.; Miras, M.C.; Barbero, C. Strong effects of counterions on the electrochemistry of poly(N-methylaniline) thin films. Polym. Int. 2002, 51, 429–433. [Google Scholar] [CrossRef]
- Lira, L.M.; Córdoba De Torresi, S.I. Conducting polymer-hydrogel composites for electrochemical release devices: Synthesis and characterization of semi-interpenetrating polyaniline- polyacrylamide networks. Electrochem. Comm. 2005, 7, 717–723. [Google Scholar] [CrossRef]
- Vashist, A.; Kaushik, A.; Ghosal, A.; Bala, J.; Nikkhah-Moshaie, R.; Wani, W.A.; Manickam, P.; Nair, M. Nanocomposite Hydrogels: Advances in Nanofillers Used for Nanomedicine. Gels 2018, 4, 75. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lu, A.; Yang, Z.; Luo, Y. Enhanced swelling and mechanical properties of P(AM-co-SMA) semi-IPN composite hydrogels by impregnation with PANI and MWNTs-COOH. Macromol. Res. 2013, 21, 376–384. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, Y.; Zhang, K. P(AAm-co-MAA) semi-IPN hybrid hydrogels in the presence of PANI and MWNTs-COOH: Improved swelling behavior and mechanical properties. J. Biomater. Sci. Polym. Ed. 2008, 9, 1503–1520. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [Green Version]
- Siraj, S.; Al-Marzouqi, A.H.; Iqbal, M.Z.; Ahmed, W. Impact of Micro Silica Filler Particle Size on Mechanical Properties of Polymeric Based Composite Material. Polymers 2022, 14, 4830. [Google Scholar] [CrossRef]
- Panteli, P.A.; Patrickios, C.S. Multiply Interpenetrating Polymer Networks: Preparation, Mechanical Properties, and Applications. Gels 2019, 5, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, D.O.; Dorneles, M.F.; Oréfice, R.L. A facile and low-cost route for producing a flexible hydrogel–PANI electrolyte/counter electrode applicable in dye-sensitized solar cells (DSSC). SN Appl. Sci. 2019, 1, 1598. [Google Scholar] [CrossRef] [Green Version]
- Asadujjaman, A.; Kent, B.; Bertin, A. Phase transition and aggregation behaviour of an UCST-type copolymer poly(acrylamide-co-acrylonitrile) in water: Effect of acrylonitrile content, concentration in solution, copolymer chain length and presence of electrolyte. Soft Matter 2017, 13, 658–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Zhu, L.; Zhao, C.; Wang, Q.; Zheng, J. A robust, one-pot synthesis of highly mechanical and recoverable double network hydrogels using thermoreversible sol-gel polysaccharide. Adv. Mater. 2013, 25, 4171–4176. [Google Scholar] [CrossRef]







| Material | Gauge Factor (GF) | Ref. |
|---|---|---|
| cPNIPAM-co-2%AMPS/PANI(NC) | 5.64 | [96] |
| cPNIPAM-co-2%AMPS/PANI(s-IPN) | 0.95 | [64] |
| PNIPAM c-Pluronic F127/PANI(NC) | 3.92 | [104] |
| cP(AAm-co-HEMA) (NC) | 11 | [106] |
| Material | Areal Capacitance F cm−2 | Specific Capacitance (F g−1) | Ref. |
|---|---|---|---|
| Prestretched cPAAm/PANI | 0.5099 | -- | [120] |
| cPAAM/HPA/AgNPs(lignin)/PANI | 0.364 | -- | [121] |
| PANI/cellulose/PAAm/PANI | 0.835 | -- | [101] |
| αCD-PAAm/PANI | -- | 315 | [122] |
| Hydrogel Matrix | PANI Incorporation Method | Mechanical Strength (kPa) | Ref. |
|---|---|---|---|
| MEO2MA-OEGMA-NIPAM | ISP * | 7210 | [102] |
| cP(AAm-co-SMA)/MWNTs-COOH | Entrapment of solid PANI | 590 | [131] |
| cP(AAm-co-MAA)/MWNT-COOH | ISP * | 800 | [132] |
| cPNIPAM-co-2%AMPS | None | 6.5 | [64] |
| cPNIPAM-co-2%AMPS | ISP (NC) | 11.3 | [64] |
| cPNIPAM-co-2%AMPS | Absorption from solution (s-IPN) | 17.3 | [64] |
| cPAAm/HPA/AgNPs(lignin) | ISP * | 130.7 | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbero, C.A. Functional Materials Made by Combining Hydrogels (Cross-Linked Polyacrylamides) and Conducting Polymers (Polyanilines)—A Critical Review. Polymers 2023, 15, 2240. https://doi.org/10.3390/polym15102240
Barbero CA. Functional Materials Made by Combining Hydrogels (Cross-Linked Polyacrylamides) and Conducting Polymers (Polyanilines)—A Critical Review. Polymers. 2023; 15(10):2240. https://doi.org/10.3390/polym15102240
Chicago/Turabian StyleBarbero, Cesar A. 2023. "Functional Materials Made by Combining Hydrogels (Cross-Linked Polyacrylamides) and Conducting Polymers (Polyanilines)—A Critical Review" Polymers 15, no. 10: 2240. https://doi.org/10.3390/polym15102240