Enzymatic Crosslinking of Amino Acids Improves the Repair Effect of Keratin on Hair Fibre
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
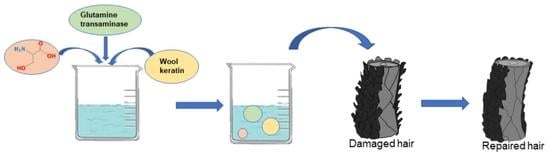
2.2. Repair Solutions
2.3. Preparation of Permed Hair Samples
2.4. Hair Repair
2.5. Characterisation of Amide Bonds (FTIR)
2.6. Determination of Hair Crystallinity (XRD)
2.7. Determination of Alkali Solubility
2.8. Assessment of Thermal Stability (DSC)
2.9. Determination of Mechanical Properties
2.10. Morphological Characterisation (SEM)
2.11. Damage Prevention Testing
2.12. Hair Care Durability Test
2.13. Moisturising Performance Test
3. Results and Discussion
3.1. Formation of Amide Bonds
3.2. Hair Crystallinity
3.3. Alkali Solubility
3.4. Thermal Stability
3.5. Mechanical Properties
3.6. Morphological Characterisation
3.7. Damage Prevention
3.8. Persistence
3.9. Moisture Retention
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kajiura, Y.; Watanabe, S.; Itou, T.; Nakamura, K.; Iida, A.; Inoue, K.; Yagi, N.; Shinohara, Y.; Amemiya, Y. Structural analysis of human hair single fibres by scanning microbeam SAXS. J. Struct. Biol. 2006, 3, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.V.R.; Dias, T.C.D.S.; Freitas, A.Z.D.; Júnior, N.D.V.; Pinto, C.A.S.D.O.; Kaneko, T.M.; Baby, A.R. Hair fiber characteristics and methods to evaluate hair physical and mechanical properties. Braz. J. Pharm. Sci. 2009, 45, 153–162. [Google Scholar] [CrossRef]
- Wolfram, L.J. Human hair: A unique physicochemical composite. J. Am. Acad. Dermatol. 2003, 6, S106–S114. [Google Scholar] [CrossRef]
- Song, K.; Xu, H.; Xie, K.; Yang, Y. Effects of chemical structures of polycarboxylic acids on molecular and performance manipulation of hair keratin. RSC Adv. 2016, 6, 58594–58603. [Google Scholar] [CrossRef]
- Tinoco, A.; Martins, M.; Cavaco-Paulo, A.; Ribeiro, A. Biotechnology of functional proteins and peptides for hair cosmetic formulations. Trends Biotechnol. 2022, 40, 591–605. [Google Scholar] [CrossRef]
- Cruz, C.F.; Azoia, N.G.; Matamá, T.; Cavaco-Paulo, A. Peptide—Protein interactions within human hair keratins. Int. J. Biol. Macromol. 2017, 101, 805–814. [Google Scholar] [CrossRef]
- Watt, I.C.; Morris, R. Factors affecting the ther-mal contraction of keratin fibres. J. Polym. Sci. Pol. 1969, 229, 34–38. [Google Scholar]
- Barba, C.; Martí, M.; Roddick-Lanzilotta, A.; Manich, A.; Carilla, J.; Parra, J.; Coderch, L. Effect of wool keratin proteins and peptides on hair water sorption kinetics. J. Therm. Anal. Calorim. 2010, 102, 43–48. [Google Scholar] [CrossRef]
- Villa, A.L.V.; Aragão, M.R.S.; Dos Santos, E.P.; Mazotto, A.M.; Zingali, R.B.; De Souza, E.P.; Vermelho, A.B. Feather keratin hydrolysates obtained from microbial keratinases: Effect on hair fiber. BMC Biotechnol. 2013, 13, 15. [Google Scholar] [CrossRef]
- Gaspar, A.L.C.; de Góes-Favoni, S.P. Action of microbial transglutaminase (MTGase) in the modification of food proteins: A review. Food Chem. 2015, 171, 315–322. [Google Scholar] [CrossRef]
- Soun, B.; Kaur, D.; Jose, S. Effect of Transglutaminase Enzyme on Physico-mechanical Properties of Rambouillet Wool Fiber. J. Nat. Fibers 2020, 17, 793–801. [Google Scholar] [CrossRef]
- Cardamone, J.M.; Phillips, J.G. Enzyme-mediated Crosslinking of Wool. Part II: Keratin and Transglutaminase. Text. Res. J. 2007, 77, 277–283. [Google Scholar] [CrossRef]
- Lin, Z. Study on mTG Enzyme-Catalyzed Reinforcement of Fragile Wool Fabrics. Ph.D. Thesis, Zhejiang Sci-Tech University, Hangzhou, China, 2016. [Google Scholar]
- Li, Z.; Xiao, J. Repair of damaged hair protein fiber by jointly using transglutaminase and keratin. Scienceasia 2021, 47, 195. [Google Scholar] [CrossRef]
- Gillespie, J.M.; Haylett, T.; Lindley, H. Evidence of homology in a high-sulphur protein fraction (SCMK-B2) of wool and hair alpha-keratins. Biochem. J. 1968, 110, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Segal, L. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- The Textile Journal Group. Total Colour Management in Textiles; Woodhead: Sawston, UK, 2006; p. 2. [Google Scholar]
- Wojciechowska, E.; Włochowicz, A.; Wesełucha-Birczyńska, A. Application of fourier-transform infrared and raman spectroscopy to study degradation of the wool fiber keratin. J. Mol. Struct. 1999, 511, 307–318. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, N.; Wang, Q.; Wang, P.; Yuan, J.; Shen, J.; Fan, X. Disulfide bond reconstruction: A novel approach for grafting of thiolated chitosan onto wool. Carbohydr. Polym. 2018, 203, 369–377. [Google Scholar] [CrossRef]
- Malinauskyte, E.; Shrestha, R.; Cornwell, P.A.; Gourion-Arsiquaud, S.; Hindley, M. Penetration of different molecular weight hydrolyzed keratins into hair fibers and their effects on the physical properties of textured hair. Int. J. Cosmet. Sci. 2021, 43, 26–37. [Google Scholar] [CrossRef]
- Fernandes, M.; Cavaco-Paulo, A. Protein disulphide isomerase-mediated grafting of cysteine-containing peptides onto over-bleached hair. Biocatal. Biotransform. 2012, 30, 10–19. [Google Scholar] [CrossRef]
- Ribeiro, A.; Matamá, T.; Cruz, C.F.; Gomes, A.C.; Cavaco-Paulo, A.M. Potential of human γD-crystallin for hair damage repair: Insights into the mechanical properties and biocompatibility. Int. J. Cosmet. Sci. 2013, 35, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Barba, C.; Scott, S.; Roddick-Lanzilotta, A.; Kelly, R.; Manich, A.M.; Parra, J.L.; Coderch, L. Restoring Important Hair Properties with Wool Keratin Proteins and Peptides. Fibers Polym. 2010, 11, 1055. [Google Scholar] [CrossRef]
- Yi, Z.R.; Hao, Z.; Li, S. Preparation of rabbit hair keratin and its application in sunscreen cosmetics. Res. Dev. Nat. Prod. 2018, 30, 120. [Google Scholar]








| Damaged Hair | K-sRS Hair Sample | K-eRS Hair Sample | K-s-eRS Hair Sample | |
|---|---|---|---|---|
| Crystallinity (%) | 21.91 | 15.01 | 16.57 | 13.76 |
| Healthy Hair | Damaged Hair | K-sRS Hair Sample | K-eRS Hair Sample | K-s-eRS Hair Sample | |
|---|---|---|---|---|---|
| Fracture stress (N) | 2.014 | 1.031 | 1.337 | 1.648 | 1.806 |
| Fracture strain (mm) | 20.73 | 9.51 | 12.87 | 15.63 | 19.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, J.; Xiao, J. Enzymatic Crosslinking of Amino Acids Improves the Repair Effect of Keratin on Hair Fibre. Polymers 2023, 15, 2210. https://doi.org/10.3390/polym15092210
Liu Y, Liu J, Xiao J. Enzymatic Crosslinking of Amino Acids Improves the Repair Effect of Keratin on Hair Fibre. Polymers. 2023; 15(9):2210. https://doi.org/10.3390/polym15092210
Chicago/Turabian StyleLiu, Yang, Jingjing Liu, and Jing Xiao. 2023. "Enzymatic Crosslinking of Amino Acids Improves the Repair Effect of Keratin on Hair Fibre" Polymers 15, no. 9: 2210. https://doi.org/10.3390/polym15092210