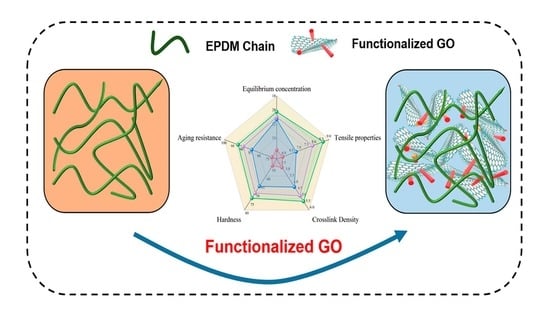
Enhancing the Anti-Migration Performance and Mechanical Properties of EPDM Insulation through Functionalized GO
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of AGO and HGO
2.3. Fabrication of EPDM Insulation Composites
2.4. Characterization
3. Results and Discussion
3.1. Characterization of GO, AGO, and HGO
3.2. Dispersion of the Fillers
3.3. Anti-Migration Performance
3.4. Thermal Analysis
3.5. Contact Angle Characterization
3.6. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, Y.W.; Li, J.; Liu, Y.; Xu, T.W. Influence of different propellant systems on ablation of EPDM insulators in overload state. Acta Astronaut. 2018, 145, 141–152. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, J.; Fang, H.; Zhang, Y.; Bai, S.; He, G. High dimensional stability and low viscous response solid propellant binder based on graphene oxide nanosheets and dual cross-linked polyurethane. Compos. Sci. Technol. 2018, 161, 124–134. [Google Scholar] [CrossRef]
- Ghosh, K.; Gaikwad, L.V.; Kalal, R.K.; Kulkarni, P.A.; Kumar, A.; Banerjee, S.; Gupta, M. Light weight HTPB-clay nanocomposites (HCN) with enhanced ablation performance as inhibition materials for composite propellant. Def. Technol. 2021, 17, 559–570. [Google Scholar] [CrossRef]
- Hoffmann, L.F.S.; Bizarria, F.C.P.; Bizarria, J.W.P. Detection of liner surface defects in solid rocket motors using multilayer perceptron neural networks. Polym. Test. 2020, 88, 106559. [Google Scholar] [CrossRef]
- Chen, J.M.; He, J.X.; Liu, L.Q.; He, Y.Z.; Huang, X.B. Effect of hybrid cross-linked polyphosphazene microspheres on ablative and mechanical properties of ethylene propylene diene monomer. J. Appl. Polym. Sci. 2021, 138, 51348. [Google Scholar] [CrossRef]
- Lu, Z.H.; Hu, Y.B.; Zhang, B.H.; Zhang, G.P.; Guo, F.; Jiang, W. Anti-migration performance of EPDM composite improved by octadecylamine-functionalized graphene oxide. J. Appl. Polym. Sci. 2022, 139, 52713. [Google Scholar] [CrossRef]
- He, Y.; Chen, Y.; Liu, C.; Huang, L.; Huang, C.; Lu, J.; Huang, H. Construction of Three-Dimensional Network Structure in Polyethylene-EPDM-Based Phase Change Materials by Carbon Nanotube with Enhanced Thermal Conductivity, Mechanical Property and Photo-Thermal Conversion Performance. Polymers 2022, 14, 2285. [Google Scholar] [CrossRef]
- Libardi, J.; Ravagnani, S.P.; Morais, A.M.F.; Cardoso, A.R. Study of plasticizer diffusion in a solid rocket motor’s bondline. J. Aerosp. Technol. Manag. 2009, 1, 223–229. [Google Scholar] [CrossRef]
- Gottlieb, L.; Bar, S. Migration of plasticizer between bonded propellant interfaces. Propell. Explos. Pyrot. 2003, 28, 12–17. [Google Scholar] [CrossRef]
- Huang, Z.P.; Nie, H.Y.; Zhang, Y.Y.; Tan, L.M.; Yin, H.L.; Ma, X.G. Migration kinetics and mechanisms of plasticizers, stabilizers at interfaces of NEPE propellant/HTPB liner/EDPM insulation. J. Hazard. Mater. 2012, 229, 251–257. [Google Scholar] [CrossRef]
- Rezaei-Vahidian, H.; Farajpour, T.; Abdollahi, M. Using an inhibitor to prevent plasticizer migration from polyurethane matrix to EPDM based substrate. Chin. J. Polym. Sci. 2019, 37, 681–686. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.; Zhang, W.; Ren, R.; Zhang, Z.; Luo, Y. Preparation of anti-migration transition layer and its application in cast-in-case solid rocket motors. J. Appl. Polym. Sci. 2021, 138, 50680. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Y.; Chen, J.; Li, Y.; Cheng, Y. Nitroglycerine migration to polyurethane used for inhibition of double-base propellants through thermal analysis techniques. J. Therm. Anal. Calorim. 2016, 125, 881–886. [Google Scholar] [CrossRef]
- Li, H.; Wei, J.; Zhang, Y.; Hu, Y.B.; Jiang, W.; Zhang, T.Y. GO/HTPB Composite Liner for Anti-Migration of Small Molecules. Def. Technol. 2021; in press. [Google Scholar] [CrossRef]
- Li, H.; Jiang, W.; Zhang, Y.; Lu, Z.; Hu, Y.; Xiao, C.; Zhang, T.; She, F. Solid propellant liner with high anti-migration and strong adhesion based on isocyanate-functionalized graphene oxide and hydroxy-terminated polybutadiene. J. Mater. Sci. 2022, 57, 14413. [Google Scholar] [CrossRef]
- Farajpour, T.; Moradi, S.; Rezaei-Vahidian, H. Synthesis of a new polyurethane-based liner modified by carbon nanotube to prevent plasticizer migration. Polym. Bull. 2022, 79, 2903–2914. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, D.; Xu, Z.; Zhang, L.; Liu, L.; Wen, S. High barrier properties against sulfur mustard of graphene oxide/butyl rubber composites. Compos. Sci. Technol. 2019, 170, 141–147. [Google Scholar] [CrossRef]
- Al-Jabareen, A.; Al-Bustami, H.; Harel, H.; Marom, G. Improving the oxygen barrier properties of polyethylene terephthalate by graphite nanoplatelets. J. Appl. Polym. Sci. 2013, 128, 1534–1539. [Google Scholar] [CrossRef]
- Lu, Z.H.; Hu, Y.B.; Zhang, B.H.; Zhang, G.P.; Guo, F.; Jiang, W. EPDM/GO composite insulation for anti-migration of plasticizers. J. Polym. Res. 2022, 29, 385. [Google Scholar] [CrossRef]
- Wei, J.; Zang, Z.; Zhang, Y.; Wang, M.; Du, J.; Tang, X. Enhanced performance of light-controlled conductive switching in hybrid cuprous oxide/reduced graphene oxide (Cu2O/rGO) nanocomposites. Opt. Lett. 2017, 42, 911–914. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, F.; Luo, Y.L.; Yu, X.; Wu, S.Z. Grafting silica onto reduced graphene oxide via hydrosilylation for comprehensive rubber applications: Molecular simulation and experimental study. Polym. Compos. 2022, 43, 5332–5343. [Google Scholar] [CrossRef]
- Li, J.N.; Yu, K.J.; Qian, K.; Cao, H.J.; Lu, X.F.; Sun, J. One-Step Synthesis of Graphene Nanoplatelets/SiO2 Hybrid Materials with Excellent Toughening Performance. Polym. Compos. 2015, 36, 907–912. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhou, M.Y.; Xia, Z.B.; Gong, Q.; Liu, X.H.; Yang, Y.; Gao, Q.W. Facile preparation of polyaniline covalently grafted to isocyanate functionalized reduced graphene oxide nanocomposite for high performance flexible supercapacitors. Colloids Surf. A 2020, 602, 125172. [Google Scholar] [CrossRef]
- Zheng, L.; Jerrams, S.; Xu, Z.C.; Zhang, L.Q.; Liu, L.; Wen, S.P. Enhanced gas barrier properties of graphene oxide/rubber composites with strong interfaces constructed by graphene oxide and sulfur. Chem. Eng. J. 2020, 383, 123100. [Google Scholar] [CrossRef]
- Hu, K.S.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Zeng, Y.; Luo, X.; Yu, K.J.; Qian, K. EMI shielding performance of phenolic-based carbon foam modified with GO/SiO2 hybrid nanomaterials. Chem. Phys. Lett. 2019, 715, 166–172. [Google Scholar] [CrossRef]
- Khaki, A.; Garmabi, H.; Javadi, A.; Yahyaee, N. Effect of crystallinity, crystal polymorphism, and graphene oxide nanosheets on the barrier properties of poly (L-lactic acid). Eur. Polym. J. 2019, 118, 53–63. [Google Scholar] [CrossRef]
- Abidin, A.S.Z.; Yusoh, K.; Jamari, S.S.; Abdullah, A.H.; Ismail, Z. Surface functionalization of graphene oxide with octadecylamine for improved thermal and mechanical properties in polybutylene succinate nanocomposite. Polym. Bull. 2018, 75, 3499–3522. [Google Scholar] [CrossRef]
- Lv, Y.; Deng, Q.; Row, K.H.; Zhu, T. Silane coupling agents modified silica and graphene oxide materials for determination of sulfamerazine and sulfameter in milk by HPLC. Food Anal. Methods 2019, 12, 687–696. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Liu, H.; Shang, S.; Song, Z.; Song, J. Properties enhancement of room temperature vulcanized silicone rubber by rosin modified aminopropyltriethoxysilane as a cross-linking agent. ACS Sustain. Chem. Eng. 2017, 5, 10002–10010. [Google Scholar] [CrossRef]
- Yin, S.; Lu, Z.; Bai, H.; Liu, X.; Li, H.; Hu, Y. Functionalized GO/Hydroxy-Terminated Polybutadiene Composites with High Anti-Migration and Ablation Resistance Performance. Polymers 2022, 14, 3315. [Google Scholar] [CrossRef] [PubMed]










| Samples | 25 °C/% | 40 °C/% | 60 °C/% | 80 °C/% |
|---|---|---|---|---|
| EPDM | 25.71 | 28.86 | 36.48 | 45.11 |
| GO/EPDM | 21.36 | 26.68 | 33.25 | 41.82 |
| AGO/EPDM | 20.28 | 24.35 | 31.17 | 40.93 |
| HGO/EPDM | 21.06 | 25.91 | 31.48 | 41.26 |
| Samples | 25 °C | 40 °C | 60 °C | 80 °C |
|---|---|---|---|---|
| EPDM | 4.10 × 10−14 | 1.73 × 10−13 | 7.12 × 10−13 | 1.54 × 10−12 |
| GO/EPDM | 2.92 × 10−14 | 1.47 × 10−13 | 5.95 × 10−13 | 1.49 × 10−12 |
| AGO/EPDM | 2.47 × 10−14 | 1.18 × 10−13 | 5.16 × 10−13 | 1.31 × 10−12 |
| HGO/EPDM | 2.60 × 10−14 | 1.43 × 10−13 | 5.90 × 10−13 | 1.42 × 10−12 |
| Samples | Ea (kJ/mol) | r2 |
|---|---|---|
| EPDM | 58.07 | 0.97 |
| GO/EPDM | 61.47 | 0.96 |
| AGO/EPDM | 63.46 | 0.98 |
| HGO/EPDM | 62.17 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Zhu, Z.; Zhang, Y.; Wang, C.; Bai, H.; Zhang, G.; Hu, Y.; Jiang, W. Enhancing the Anti-Migration Performance and Mechanical Properties of EPDM Insulation through Functionalized GO. Polymers 2023, 15, 1731. https://doi.org/10.3390/polym15071731
Lu Z, Zhu Z, Zhang Y, Wang C, Bai H, Zhang G, Hu Y, Jiang W. Enhancing the Anti-Migration Performance and Mechanical Properties of EPDM Insulation through Functionalized GO. Polymers. 2023; 15(7):1731. https://doi.org/10.3390/polym15071731
Chicago/Turabian StyleLu, Zhehong, Ziqiang Zhu, Yulong Zhang, Chenyang Wang, Haoran Bai, Guangpu Zhang, Yubing Hu, and Wei Jiang. 2023. "Enhancing the Anti-Migration Performance and Mechanical Properties of EPDM Insulation through Functionalized GO" Polymers 15, no. 7: 1731. https://doi.org/10.3390/polym15071731