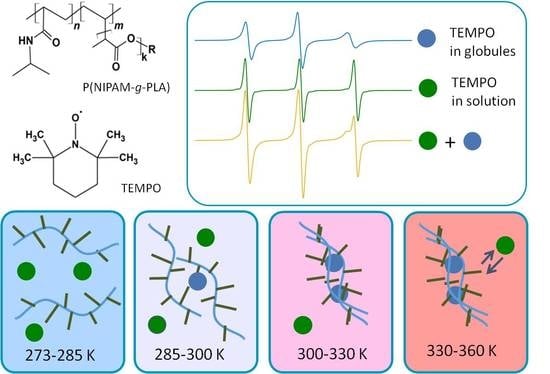
Structure and Dynamics of Inhomogeneities in Aqueous Solutions of Graft Copolymers of N-Isopropylacrylamide with Lactide (P(NIPAM-graft-PLA)) by Spin Probe EPR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substances
2.2. Preparation of Solutions
2.3. EPR Spectroscopy
2.4. EPR Spectra Treatment and Simulation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. EPR Spectra Simulation Details
| Polymer, Concentration | T, K | giso | aiso, mT | tcorr,x, ns | tcorr,y, ns | tcorr,z, ns | tcorr,iso, ns |
|---|---|---|---|---|---|---|---|
| I, 10 wt% | 353 | 2.00615 | 1.60 | 21.9 | 1.9 | 0.3 | 0.7 |
| III, 10 wt% | 363 | 2.00620 | 1.62 | 17.8 | 0.2 | 1.0 | 0.6 |
| 353 | 2.00620 | 1.60 | 25.1 | 0.3 | 1.8 | 0.7 | |
| 343 | 2.00617 | 1.60 | 25.1 | 0.4 | 2.0 | 0.9 | |
| III, 5 wt% | 363 | 2.00610 | 1.62 | 17.8 | 0.3 | 1.1 | 0.7 |
| 353 | 2.00607 | 1.60 | 25.1 | 0.3 | 1.8 | 0.8 | |
| 343 | 2.00607 | 1.60 | 25.1 | 0.4 | 2.1 | 1.1 | |
| IV, 10 wt% | 363 | 2.00613 | 1.62 | 17.8 | 0.3 | 1.1 | 0.7 |
| 353 | 2.00613 | 1.60 | 17.8 | 0.3 | 1.9 | 0.8 | |
| 343 | 2.00607 | 1.60 | 18.6 | 0.4 | 2.2 | 1.1 | |
| IV, 5 wt% | 363 | 2.00597 | 1.62 | 17.8 | 0.3 | 1.1 | 0.7 |
| 353 | 2.00597 | 1.60 | 17.8 | 0.3 | 1.9 | 0.8 | |
| 343 | 2.00590 | 1.60 | 18.6 | 0.4 | 2.2 | 1.1 | |
| V, 5 wt% | 363 | 2.00610 | 1.61 | 9.8 | 0.2 | 1.0 | 0.5 |
| 353 | 2.00607 | 1.60 | 16.2 | 0.3 | 1.7 | 0.7 | |
| 343 | 2.00607 | 1.58 | 16.6 | 0.4 | 2.3 | 0.9 |
| II, 10 wt% solution | |||||||
| T, K | Type A | Type B | |||||
| giso | aiso, mT | tcorr,iso, ps | giso | aiso, mT | tcorr,iso, ns | XB, % | |
| 360 | 2.00591 | 1.72 | 10.0 | 2.00609 | 1.62 | 0.7 | 68.1 |
| 350 | 2.00590 | 1.72 | 10.0 | 2.00611 | 1.60 | 0.8 | 68.2 |
| 340 | 2.0059 | 1.72 | 10.0 | 2.00606 | 1.59 | 1.1 | 68.1 |
| 330 | 2.00588 | 1.73 | 10.0 | 2.00602 | 1.58 | 1.3 | 67.7 |
| 320 | 2.00586 | 1.73 | 10.0 | 2.00598 | 1.57 | 1.7 | 66.9 |
| 315 | 2.00586 | 1.73 | 10.0 | 2.00595 | 1.57 | 1.8 | 65.8 |
| 310 | 2.00585 | 1.73 | 10.0 | 2.00588 | 1.57 | 1.9 | 65.5 |
| 308 | 2.00585 | 1.73 | 10.0 | 2.00589 | 1.57 | 1.9 | 63.4 |
| 306 | 2.00585 | 1.73 | 10.0 | 2.00588 | 1.57 | 1.9 | 58.5 |
| 304 | 2.00586 | 1.73 | 10.0 | 2.00587 | 1.57 | 1.8 | 51.3 |
| 303 | 2.00584 | 1.73 | 10.0 | 2.00587 | 1.57 | 1.8 | 48.3 |
| 302 | 2.00585 | 1.73 | 10.0 | 2.00587 | 1.57 | 1.7 | 42.4 |
| 360 | 2.00591 | 1.72 | 10.0 | 2.00609 | 1.62 | 0.9 | 68.1 |
| 350 | 2.00590 | 1.72 | 10.0 | 2.00611 | 1.60 | 0.8 | 68.2 |
| III, 10 wt% solution | |||||||
| T, K | Type A | Type B | |||||
| giso | aiso, mT | tcorr,iso, ps | giso | aiso, mT | tcorr,iso, ns | XB, % | |
| 363 | 2.00591 | 1.71 | 10.0 | 2.00609 | 1.62 | 0.6 | 78.6 |
| 353 | 2.00590 | 1.72 | 10.0 | 2.00608 | 1.60 | 0.8 | 75.6 |
| 343 | 2.00590 | 1.72 | 10.0 | 2.00605 | 1.60 | 1.0 | 76.6 |
| 333 | 2.00589 | 1.72 | 10.0 | 2.00602 | 1.59 | 1.2 | 77.0 |
| 323 | 2.00587 | 1.73 | 10.0 | 2.00598 | 1.58 | 1.5 | 73.3 |
| 313 | 2.00586 | 1.73 | 10.0 | 2.00596 | 1.57 | 1.9 | 69.3 |
| 308 | 2.00585 | 1.73 | 10.0 | 2.00588 | 1.57 | 1.8 | 64.9 |
| 306 | 2.00585 | 1.73 | 10.0 | 2.00586 | 1.57 | 1.8 | 61.5 |
| 305 | 2.00585 | 1.73 | 10.0 | 2.00584 | 1.57 | 1.8 | 58.4 |
| 304 | 2.00583 | 1.73 | 10.0 | 2.00581 | 1.57 | 1.6 | 51.3 |
| 302 | 2.00580 | 1.73 | 10.0 | 2.00570 | 1.57 | 1.6 | 35.5 |
| III, 5 wt% solution | |||||||
| T, K | Type A | Type B | |||||
| giso | aiso, mT | tcorr,iso, ps | giso | aiso, mT | tcorr,iso, ns | XB, % | |
| 363 | 2.00584 | 1.71 | 10.0 | 2.00602 | 1.62 | 0.6 | 63.8 |
| 353 | 2.00583 | 1.72 | 10.0 | 2.00601 | 1.60 | 0.8 | 58.9 |
| 343 | 2.00582 | 1.72 | 10.0 | 2.00596 | 1.60 | 1.0 | 58.4 |
| 333 | 2.00581 | 1.72 | 10.0 | 2.00591 | 1.59 | 1.3 | 55.4 |
| 323 | 2.00580 | 1.73 | 10.0 | 2.00585 | 1.58 | 1.6 | 53.9 |
| 313 | 2.00580 | 1.73 | 10.0 | 2.00580 | 1.56 | 2.0 | 49.0 |
| 308 | 2.00580 | 1.73 | 10.0 | 2.00574 | 1.56 | 2.0 | 44.3 |
| 306 | 2.00579 | 1.73 | 10.0 | 2.00574 | 1.56 | 2.0 | 42.6 |
| 305 | 2.00579 | 1.73 | 10.0 | 2.00574 | 1.56 | 2.0 | 39.9 |
| 304 | 2.00579 | 1.73 | 10.0 | 2.00574 | 1.56 | 2.0 | 31.7 |
| 302 | 2.00579 | 1.73 | 10.0 | 2.00574 | 1.56 | 2.0 | 27.5 |
| IV, 10 wt% solution | |||||||
| T, K | Type A | Type B | |||||
| giso | aiso, mT | tcorr,iso, ps | giso | aiso, mT | tcorr,iso, ns | XB, % | |
| 363 | 2.00586 | 1.71 | 10.0 | 2.00608 | 1.62 | 0.6 | 63.6 |
| 360 | 2.00586 | 1.71 | 10.0 | 2.00607 | 1.62 | 0.6 | 64.0 |
| 350 | 2.00585 | 1.72 | 10.0 | 2.00607 | 1.60 | 0.8 | 66.0 |
| 340 | 2.00585 | 1.72 | 10.0 | 2.00602 | 1.59 | 1.0 | 66.9 |
| 330 | 2.00584 | 1.72 | 10.0 | 2.00601 | 1.59 | 1.3 | 65.5 |
| 320 | 2.00584 | 1.73 | 10.0 | 2.00596 | 1.58 | 1.6 | 66.4 |
| 310 | 2.00584 | 1.73 | 10.0 | 2.00594 | 1.58 | 1.9 | 62.9 |
| 300 | 2.00584 | 1.73 | 12.4 | 2.00584 | 1.58 | 1.9 | 37.7 |
| 295 | 2.00585 | 1.73 | 18.7 | 2.00577 | 1.58 | 1.9 | 22.1 |
| IV, 5 wt% solution | |||||||
| T, K | Type A | Type B | |||||
| giso | aiso, mT | tcorr,iso, ps | giso | aiso, mT | tcorr,iso, ns | XB, % | |
| 363 | 2.00594 | 1.71 | 10.0 | 2.00613 | 1.62 | 0.7 | 55.3 |
| 353 | 2.00593 | 1.72 | 10.0 | 2.00613 | 1.60 | 0.8 | 54.3 |
| 343 | 2.00592 | 1.72 | 10.0 | 2.00607 | 1.60 | 1.0 | 55.3 |
| 333 | 2.00591 | 1.72 | 10.0 | 2.00603 | 1.58 | 1.3 | 56.3 |
| 323 | 2.00590 | 1.73 | 10.0 | 2.00600 | 1.56 | 1.7 | 55.2 |
| 313 | 2.00589 | 1.73 | 10.0 | 2.00595 | 1.56 | 2.0 | 51.5 |
| 308 | 2.00590 | 1.73 | 10.0 | 2.00592 | 1.56 | 2.1 | 46.7 |
| 305 | 2.00589 | 1.73 | 10.0 | 2.00588 | 1.56 | 2.1 | 38.9 |
| 302 | 2.00591 | 1.73 | 10.0 | 2.00587 | 1.56 | 2.0 | 32.4 |
| 300 | 2.00591 | 1.73 | 11.0 | 2.00587 | 1.56 | 2.0 | 27.8 |
| 298 | 2.00588 | 1.73 | 12.2 | 2.00587 | 1.56 | 2.0 | 23.3 |
| 295 | 2.00593 | 1.73 | 12.2 | 2.00587 | 1.56 | 2.0 | 19.0 |
| 292 | 2.00590 | 1.73 | 12.2 | 2.00587 | 1.56 | 0.5 | 12.7 |
| 287 | 2.00586 | 1.73 | 12.2 | 2.00587 | 1.56 | 0.8 | 11.5 |
| 282 | 2.00590 | 1.74 | 12.2 | 2.00587 | 1.56 | 0.2 | 7.8 |
| V, 5 wt% solution | |||||||
| T, K | Type A | Type B | |||||
| giso | aiso, mT | tcorr,iso, ps | giso | aiso, mT | tcorr,iso, ns | XB, % | |
| 343 | 2.00585 | 1.72 | 10.0 | 2.00603 | 1.58 | 1.0 | 54.1 |
| 333 | 2.00585 | 1.72 | 10.0 | 2.00595 | 1.58 | 1.4 | 54.0 |
| 323 | 2.00583 | 1.73 | 10.0 | 2.00587 | 1.56 | 1.9 | 56.1 |
| 313 | 2.00577 | 1.73 | 10.0 | 2.00587 | 1.56 | 1.9 | 49.8 |
| 308 | 2.00576 | 1.73 | 10.0 | 2.00587 | 1.56 | 1.9 | 42.7 |
| 306 | 2.00576 | 1.73 | 10.0 | 2.00587 | 1.56 | 2.2 | 41.0 |
| 302 | 2.00575 | 1.73 | 10.0 | 2.00587 | 1.56 | 0.8 | 28.5 |
| 300 | 2.00575 | 1.73 | 11.3 | 2.00587 | 1.56 | 0.8 | 24.3 |
| 298 | 2.00575 | 1.73 | 11.3 | 2.00587 | 1.56 | 0.8 | 19.2 |
| 296 | 2.00576 | 1.73 | 11.3 | 2.00587 | 1.56 | 1.2 | 19.6 |
| T, K | aiso, mT | tcorr,iso, ps |
| 273 | 1.74 | 12.1 |
| 283 | 1.74 | 10.9 |
| 293 | 1.73 | 10.0 |
| 303 | 1.73 | 10.0 |
| 313 | 1.73 | 10.0 |
| 323 | 1.73 | 10.0 |
| 333 | 1.72 | 10.0 |
| 343 | 1.72 | 10.0 |
| 353 | 1.72 | 10.0 |
References
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New Directions in Thermoresponsive Polymers. Chem. Soc. Rev. 2013, 42, 7214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive Polymers with Lower Critical Solution Temperature: From Fundamental Aspects and Measuring Techniques to Recommended Turbidimetry Conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Otake, K.; Inomata, H.; Konno, M.; Saito, S. Thermal Analysis of the Volume Phase Transition with N-Isopropylacrylamide Gels. Macromolecules 1990, 23, 283–289. [Google Scholar] [CrossRef]
- Ksendzov, E.A.; Nikishau, P.A.; Zurina, I.M.; Presniakova, V.S.; Timashev, P.; Rochev, Y.A.; Kotova, S.; Kostjuk, S.V. Graft Copolymers of N-Isopropylacrylamide with Poly(d,l-Lactide) or Poly(ε-Caprolactone) Macromonomers: A Promising Class of Thermoresponsive Polymers with a Tunable LCST. ACS Appl. Polym. Mater. 2022, 4, 1344–1357. [Google Scholar] [CrossRef]
- Kurzbach, D.; Junk, M.J.N.; Hinderberger, D. Nanoscale Inhomogeneities in Thermoresponsive Polymers. Macromol. Rapid Commun. 2013, 34, 119–134. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and PH-Responsive Polymers in Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Y.; Hu, X.; Gong, H.; Li, R.; Cox, H.; Zhang, J.; Waigh, T.A.; Xu, H.; Lu, J.R. Reversible Thermoresponsive Peptide–PNIPAM Hydrogels for Controlled Drug Delivery. Biomacromolecules 2019, 20, 3601–3610. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Liu, S.-Q.; Heng, P.W.-S.; Yang, Y.-Y. Evaluating Proteins Release from, and Their Interactions with, Thermosensitive Poly (N-Isopropylacrylamide) Hydrogels. J. Control. Release 2005, 102, 361–372. [Google Scholar] [CrossRef]
- Agnihotri, P.; Sangeeta; Aery, S.; Dan, A. Temperature- and PH-Responsive Poly(N-Isopropylacrylamide-Co-Methacrylic Acid) Microgels as a Carrier for Controlled Protein Adsorption and Release. Soft Matter 2021, 17, 9595–9606. [Google Scholar] [CrossRef]
- Kanazawa, H.; Okano, T. Temperature-Responsive Chromatography for the Separation of Biomolecules. J. Chromatogr. A 2011, 1218, 8738–8747. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cao, Y.; Song, X.; Zhang, Z.; Xiao, C.; He, C.; Chen, X. PH- and Thermo-Responsive Poly(N-Isopropylacrylamide-Co-Acrylic Acid Derivative) Copolymers and Hydrogels with LCST Dependent on PH and Alkyl Side Groups. J. Mater. Chem. B 2013, 1, 5578–5587. [Google Scholar] [CrossRef] [PubMed]
- Efremov, Y.M.; Zurina, I.M.; Presniakova, V.S.; Kosheleva, N.V.; Butnaru, D.V.; Svistunov, A.A.; Rochev, Y.A.; Timashev, P.S. Mechanical Properties of Cell Sheets and Spheroids: The Link between Single Cells and Complex Tissues. Biophys. Rev. 2021, 13, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, J.; Gao, Y.; Yang, L. Cell Sheet Technology: A Promising Strategy in Regenerative Medicine. Cytotherapy 2019, 21, 3–16. [Google Scholar] [CrossRef]
- Takezawa, T.; Mori, Y.; Yoshizato, K. Cell Culture on a Thermo-Responsive Polymer Surface. Nat. Biotechnol. 1990, 8, 854–856. [Google Scholar] [CrossRef]
- Nash, M.E.; Fan, X.; Carroll, W.M.; Gorelov, A.V.; Barry, F.P.; Shaw, G.; Rochev, Y.A. Thermoresponsive Substrates Used for the Expansion of Human Mesenchymal Stem Cells and the Preservation of Immunophenotype. Stem Cell Rev. Rep. 2013, 9, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N-Isopropylacrylamide). J. Macromol. Sci. Part A—Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Frolova, A.; Ksendzov, E.; Kostjuk, S.; Efremov, Y.; Solovieva, A.; Rochev, Y.; Timashev, P.; Kotova, S. Thin Thermoresponsive Polymer Films for Cell Culture: Elucidating an Unexpected Thermal Phase Behavior by Atomic Force Microscopy. Langmuir 2021, 37, 11386–11396. [Google Scholar] [CrossRef]
- Ikada, Y.; Tsuji, H. Biodegradable Polyesters for Medical and Ecological Applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Lee, B.H.; Vernon, B. Copolymers OfN-Isopropylacrylamide, HEMA-Lactate and Acrylic Acid with Time-Dependent Lower Critical Solution Temperature as a Bioresorbable Carrier. Polym. Int. 2005, 54, 418–422. [Google Scholar] [CrossRef]
- Tebong Mbah, V.; Pertici, V.; Lacroix, C.; Verrier, B.; Stipa, P.; Gigmes, D.; Trimaille, T. A Sacrificial PLA Block Mediated Route to Injectable and Degradable PNIPAAm-Based Hydrogels. Polymers 2020, 12, 925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertici, V.; Pin-Barre, C.; Rivera, C.; Pellegrino, C.; Laurin, J.; Gigmes, D.; Trimaille, T. Degradable and Injectable Hydrogel for Drug Delivery in Soft Tissues. Biomacromolecules 2019, 20, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.M.; De la Hoz Siegler, H. Tuning the Properties of PNIPAm-Based Hydrogel Scaffolds for Cartilage Tissue Engineering. Polymers 2021, 13, 3154. [Google Scholar] [CrossRef] [PubMed]
- Mader, K. Non-Invasive in Vivo Characterization of Release Processes in Biodegradable Polymers by Low-Frequency Electron Paramagnetic Resonance Spectroscopy. Biomaterials 1996, 17, 457–461. [Google Scholar] [CrossRef]
- Hinderberger, D. EPR Spectroscopy in Polymer Science. EPR Spectrosc. 2011, 321, 67–89. [Google Scholar]
- Zubanova, E.M.; Kostjuk, S.V.; Timashev, P.S.; Rochev, Y.A.; Kokorin, A.I.; Melnikov, M.Y.; Golubeva, E.N. Inhomogeneities in PNIPAM Aqueous Solutions: The Inside View by Spin Probe EPR Spectroscopy. Polymers 2021, 13, 3829. [Google Scholar] [CrossRef]
- Kurzbach, D.; Schömer, M.; Wilms, V.S.; Frey, H.; Hinderberger, D. How Structure-Related Collapse Mechanisms Determine Nanoscale Inhomogeneities in Thermoresponsive Polymers. Macromolecules 2012, 45, 7535–7548. [Google Scholar] [CrossRef]
- Caragheorgheopol, A.; Caldararu, H.; Dragutan, I.; Joela, H.; Brown, W. Micellization and Micellar Structure of a Poly(Ethylene Oxide)/Poly(Propylene Oxide)/Poly(Ethylene Oxide) Triblock Copolymer in Water Solution, as Studied by the Spin Probe Technique. Langmuir 1997, 13, 6912–6921. [Google Scholar] [CrossRef]
- Caldararu, H.; Caragheorgheopol, A.; Dimonie, M.; Donescu, D.; Dragutan, I.; Marinescu, N. Structure of Reversed Micelles In the Cyclohexane/Polyoxyethylene(4)Nonylphenol/Water System, As Studied by Spin Probe Technique. J. Phys. Chem. 1992, 96, 7109–7115. [Google Scholar] [CrossRef]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The Chemistry and Biology of Nitroxide Compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef] [Green Version]
- Kokorin, A. (Ed.) Nitroxides—Theory, Experiment and Applications; InTech: London, UK, 2012; ISBN 978-953-51-0722-4. [Google Scholar]
- Stoll, S.; Schweiger, A. EasySpin, a Comprehensive Software Package for Spectral Simulation and Analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.J.; Freed, J.H. Calculating Slow Motional Magnetic Resonance Spectra. In Spin Labeling; Springer: Boston, MA, USA, 1989; pp. 1–76. [Google Scholar]
- Freed, J.H.; Fraenkel, G.K. Theory of Linewidths in Electron Spin Resonance Spectra. J. Chem. Phys. 1963, 39, 326–348. [Google Scholar] [CrossRef]
- Salikhov, K.M. Current State of the Spin Exchange Theory in Dilute Solutions of Paramagnetic Particles. New Paradigm of Spin Exchange and Its Manifestations in EPR Spectroscopy. Phys.-Uspekhi 2019, 62, 951–975. [Google Scholar] [CrossRef]
- Ellison, W.J. Permittivity of Pure Water, at Standard Atmospheric Pressure, over the Frequency Range 0–25 THz and the Temperature Range 0–100 °C. J. Phys. Chem. Ref. Data 2007, 36, 1–18. [Google Scholar] [CrossRef]
- Kecki, Z.; Łyczkowski, B.; Kołodziejski, W. Critical Comparison of Empirical Systems Used to Describe Solvent Properties. J. Solution Chem. 1986, 15, 413–422. [Google Scholar] [CrossRef]
- Dewez, J.-L.; Lhoest, J.-B.; Detrait, E.; Berger, V.; Dupont-Gillain, C.C.; Vincent, L.-M.; Schneider, Y.-J.; Bertrand, P.; Rouxhet, P.G. Adhesion of Mammalian Cells to Polymer Surfaces: From Physical Chemistry of Surfaces to Selective Adhesion on Defined Patterns. Biomaterials 1998, 19, 1441–1445. [Google Scholar] [CrossRef]
- Dewez, J.-L.; Doren, A.; Schneider, Y.-J.; Rouxhet, P.G. Competitive Adsorption of Proteins: Key of the Relationship between Substratum Surface Properties and Adhesion of Epithelial Cells. Biomaterials 1999, 20, 547–559. [Google Scholar] [CrossRef]
- García-Peñas, A.; Biswas, C.S.; Liang, W.; Wang, Y.; Yang, P.; Stadler, F.J. Effect of Hydrophobic Interactions on Lower Critical Solution Temperature For. Polymers 2019, 11, 991. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Liu, C.; Zhao, K. Concentration Dependent Phase Behavior and Collapse Dynamics of PNIPAM Microgel by Dielectric Relaxation. Phys. Chem. Chem. Phys. 2017, 19, 15433–15443. [Google Scholar] [CrossRef]
- Stoll, S. Computational Modeling and Least-Squares Fittingof EPR Spectra. Handb. Multifrequency Electron. Paramagn. Reson. Data Tech. 2014, 3, 69–138. [Google Scholar] [CrossRef]
- Lebedev, Y.S.; Grinberg, O.Y.; Dubinsky, A.A.; Poluektov, O.G. Investigation of Spin Labels and Probes by Millimeter Band EPR. In Bioactive Spin Labels; Springer: Berlin/Heidelberg, Germany, 1992; pp. 227–278. [Google Scholar]
- Bogdanov, A.V.; Vorobiev, A.K. Orientation Order and Rotation Mobility of Nitroxide Biradicals Determined by Quantitative Simulation of EPR Spectra. Phys. Chem. Chem. Phys. 2016, 18, 31144–31153. [Google Scholar] [CrossRef] [PubMed]






| No. | Polymer | Mn, kDa | Đ | VPTT (DSC/Turbidimetry), K |
|---|---|---|---|---|
| I | PNIPAM | 107.6 | 2.03 | 305/305 |
| II | P(NIPAM-g-PLA-MA600) 97:3 | 23.5 | 1.25 | 304/299 |
| III | P(NIPAM-g-PLA-MA1200) 97:3 | 25.7 | 1.23 | 305/300 |
| IV | P(NIPAM-g-PLA-MA600) 91:9 | 27.7 | 1.23 | 301/295 |
| V | P(NIPAM-g-PLA-MA1200) 83:17 | 30.8 | 1.23 | 302/300 |
| System, Concentration | Tsim, K | aiso, mT | tcorr,iso, ps | Tstart, K | VPTT (DSC/Turbidimetry), K |
|---|---|---|---|---|---|
| TEMPO/PBS | 273 | 1.74 | 12 | - | -/- |
| I, 10 wt% 1 | 295 | 1.73 | 11 | 305 ± 1 | 305/305 |
| III, 5 wt% | 281 | 1.74 | 22 | 301 ± 2 | 305/300 |
| IV, 5 wt% | 277 | 1.74 | 12 | 298 ± 3 | 301/- |
| V, 5 wt% | 276 | 1.74 | 29 | 298 ± 2 | 302/300 |
| II, 10 wt% | 295 | 1.73 | 30 | 299 ± 1 | - |
| III, 10 wt% | 273 | 1.74 | 30 | 298 ± 1 | - |
| IV, 10 wt% | 276 | 1.74 | 50 | 298 ± 1 | - |
| System | Concentration | Tglob, K |
|---|---|---|
| I | 10 wt% | 302 |
| III | 5 wt% | 298 |
| III | 10 wt% | 296 |
| IV | 5 wt% | 293 |
| IV | 10 wt% | 294 |
| V | 5 wt% | 286 |
| Polymer | Concentration | aiso, mT | tcorr,x, ns | tcorr,y, ns | tcorr,z, ns | tcorr,iso, ns |
|---|---|---|---|---|---|---|
| I 1,2 | 10 wt% | 1.60 | 25 | 0.3 | 1.9 | 0.9 |
| II 1 | 10 wt% | 1.59 | 25 | 0.4 | 1.9 | 1.1 |
| III | 10 wt% | 1.60 | 25 | 0.4 | 2.0 | 0.9 |
| III | 5 wt% | 1.60 | 25 | 0.4 | 2.1 | 1.1 |
| IV | 10 wt% | 1.60 | 19 | 0.4 | 2.2 | 1.1 |
| IV | 5 wt% | 1.60 | 19 | 0.4 | 2.2 | 1.1 |
| V | 5 wt% | 1.58 | 17 | 0.4 | 2.3 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubanova, E.M.; Ivanova, T.A.; Ksendzov, E.A.; Kostjuk, S.V.; Timashev, P.S.; Melnikov, M.Y.; Golubeva, E.N. Structure and Dynamics of Inhomogeneities in Aqueous Solutions of Graft Copolymers of N-Isopropylacrylamide with Lactide (P(NIPAM-graft-PLA)) by Spin Probe EPR Spectroscopy. Polymers 2022, 14, 4746. https://doi.org/10.3390/polym14214746
Zubanova EM, Ivanova TA, Ksendzov EA, Kostjuk SV, Timashev PS, Melnikov MY, Golubeva EN. Structure and Dynamics of Inhomogeneities in Aqueous Solutions of Graft Copolymers of N-Isopropylacrylamide with Lactide (P(NIPAM-graft-PLA)) by Spin Probe EPR Spectroscopy. Polymers. 2022; 14(21):4746. https://doi.org/10.3390/polym14214746
Chicago/Turabian StyleZubanova, Ekaterina M., Tatiana A. Ivanova, Evgenii A. Ksendzov, Sergei V. Kostjuk, Peter S. Timashev, Mikhail Ya. Melnikov, and Elena N. Golubeva. 2022. "Structure and Dynamics of Inhomogeneities in Aqueous Solutions of Graft Copolymers of N-Isopropylacrylamide with Lactide (P(NIPAM-graft-PLA)) by Spin Probe EPR Spectroscopy" Polymers 14, no. 21: 4746. https://doi.org/10.3390/polym14214746