Nano-Hydroxyapatite from White Seabass Scales as a Bio-Filler in Polylactic Acid Biocomposite: Preparation and Characterization
Abstract
:1. Introduction
2. Materials and Methods
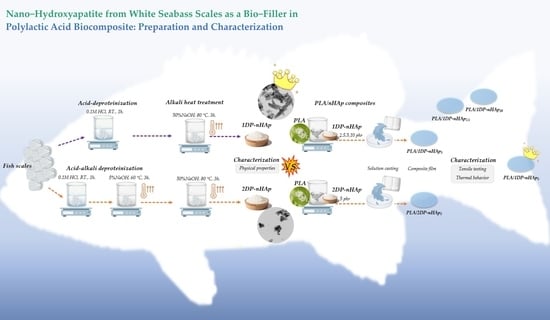
2.1. Preparation of nHAp Powder from White Sea Bass Scales
2.2. Preparation of PLA/nHAp Composite Films
2.3. Characterization of nHAp Powder
2.4. Characterization of PLA/nHAp Composite Films
2.5. Preparation of PLA/nHAp Fibers by Electrospinning Technique and Their Electrospinnability
3. Results and Discussion
3.1. Characterization of nHAp Powder
3.2. Characterization of PLA/nHAp Composite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kodali, D.; Hembrick-Holloman, V.; Gunturu, D.R.; Samuel, T.; Jeelani, S.; Rangari, V.K. Influence of Fish Scale-Based Hydroxyapatite on Forcespun Polycaprolactone Fiber Scaffolds. ACS Omega 2022, 7, 8323–8335. [Google Scholar] [CrossRef] [PubMed]
- Ideia, P.; Degli Esposti, L.; Miguel, C.C.; Adamiano, A.; Iafisco, M.; Castilho, P.C. Extraction and characterization of hydroxyapatite-based materials from grey triggerfish skin and black scabbardfish bones. Int. J. Appl. Ceram. 2021, 18, 235–243. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Nano-size hydroxyapatite extracted from tilapia scale using alkaline heat treatment method. Mater. Today Proc. 2020, 29, 218–222. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alshatwi, A.A. Simultaneous fabrication of carbon nanodots and hydroxyapatite nanoparticles from fish scale for biomedical applications. Mater. Sci. Eng. C 2020, 117, 111313. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Dextran-Coated Zinc-Doped Hydroxyapatite for Biomedical Applications. Polymers 2019, 11, 886. [Google Scholar] [CrossRef] [Green Version]
- Kaimonov, M.; Safronova, T.; Shatalova, T.; Filippov, Y.; Tikhomirova, I.; Sergeev, N. Composite Ceramics in the in the Na2O–CaO–SiO2–P2O5 System Obtained from Pastes including Hydroxyapatite and an Aqueous Solution of Sodium Silicate. Ceramics 2022, 5, 550–561. [Google Scholar] [CrossRef]
- Bernardo, M.P.; da Silva, B.C.R.; Hamouda, A.E.I.; de Toledo, M.A.S.; Schalla, C.; Rütten, S.; Sechi, A. PLA/Hydroxyapatite scaffolds exhibit in vitro immunological inertness and promote robust osteogenic differentiation of human mesenchymal stem cells without osteogenic stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef]
- Sathiskumar, S.; Vanaraj, S.; Sabarinathan, D.; Bharath, S.; Sivarasan, G.; Arulmani, S.; Ponnusamy, V.K. Green synthesis of biocompatible nanostructured hydroxyapatite from Cirrhinus mrigala fish scale—A biowaste to biomaterial. Ceram. Int. 2019, 45, 7804–7810. [Google Scholar] [CrossRef]
- Qin, D.; Bi, S.; You, X.; Wang, M.; Cong, X.; Yuan, C.; Chen, X.G. Development and application of fish scale wastes as versatile natural biomaterials. J. Chem. Eng. 2022, 428, 131102. [Google Scholar] [CrossRef]
- Ibañez, A.; Cowx, I.; O’Higgins, P. Variation in elasmoid fish scale patterns is informative with regard to taxon and swimming mode. Zool. J. Linn. Soc. 2009, 155, 834–844. [Google Scholar] [CrossRef]
- Kara, A.; Tamburaci, S.; Tihminlioglu, F.; Havitcioglu, H. Bioactive fish scale incorporated chitosan biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 130, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Irwansyah, F.S.; Noviyanti, A.R.; Eddy, D.R.; Risdiana, R. Green Template-Mediated Synthesis of Biowaste Nano-Hydroxyapatite: A Systematic Literature Review. Molecules 2022, 27, 5586. [Google Scholar] [CrossRef] [PubMed]
- Negrila, C.C.; Predoi, M.V.; Iconaru, S.L.; Predoi, D. Development of Zinc-Doped Hydroxyapatite by Sol-Gel Method for Medical Applications. Molecules 2018, 23, 2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Li, M.; Mo, H.; Chen, C.; Chen, K. Effects of Substitution Ratios of Zinc-Substituted Hydroxyapatite on Adsorption and Desorption Behaviors of Bone Morphogenetic Protein-2. Int. J. Mol. Sci. 2022, 23, 10144. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Luklinska, Z.B.; Clarke, R.L.; Davy, K.W.M. Hydroxyapatite as a filler for dental composite materials: Mechanical properties and in vitro bioactivity of composites. J Mater Sci Mater Med 2001, 12, 565–573. [Google Scholar] [CrossRef]
- Gibson, I.R. 1.3.4A—Natural and Synthetic Hydroxyapatites. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Publisher: Cambridge, MA, USA, 2020; pp. 307–317. [Google Scholar]
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef] [Green Version]
- Chittara, Y. Effect of Calcination Temperature on Quality of Hydroxyapatite that fabricated from Fish Scale Biowaste. In Proceedings of the Twenty Third International Conference on Processing and Fabrication of Advanced Materials XXIII, Indian Institute of Technology Roorkee, Roorkee, India, 5–7 December 2014. [Google Scholar]
- Kongsri, S.; Janpradit, K.; Buapa, K.; Techawongstien, S.; Chanthai, S. Nanocrystalline hydroxyapatite from fish scale waste: Preparation, characterization and application for selenium adsorption in aqueous solution. Chem. Eng. J. 2013, 215–216, 522–532. [Google Scholar] [CrossRef]
- Majhool, A.; Zainol, I.; Jaafar, C.; Mudhafar, M.; Alsailawi, H.A.; Asaad, A.; Mezaal, F. Preparation of Fish Scales Hydroxyapatite (FsHAp) for Potential Use as Fillers in Polymer. J. Chem. Chem. Eng. 2019, 13, 62–75. [Google Scholar]
- Department of Fisheries. Available online: https://www4.fisheries.go.th/local/file_document/20220916111633_1_file.pdf (accessed on 21 September 2022).
- Ranjbar Mohammadi Bonab, M.; Shakoori, P.; Arab, Z. Design and characterization of keratin/PVA-PLA nanofibers containing hybrids of nanofibrillated chitosan/ZnO nanoparticles. Int. J. Biol. Macromol. 2021, 187, 554–565. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Kanani, N.; Alfirano; Rahmayetty. Development of polylactic acid (PLA) bio-composite films reinforced with bacterial cellulose nanocrystals (BCNC) without any surface modification. J. Dispers. Sci. Technol. 2020, 41, 1488–1495. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Sa, Y.; Guo, Y.; Feng, X.; Wang, M.; Li, P.; Gao, Y.; Jiang, T. Are different crystallinity-index-calculating methods of hydroxyapatite efficient and consistent? New J. Chem. 2017, 41, 5723–5731. [Google Scholar] [CrossRef]
- Rattan, S.; Fawcett, D.; Poinern, G.E.J. Williamson-Hall based X-ray peak profile evaluation and nano-structural characterization of rod-shaped hydroxyapatite powder for potential dental restorative procedures. AIMS Mater. Sci. 2021, 8, 359–372. [Google Scholar] [CrossRef]
- Grunenwald, A.; Keyser, C.; Sautereau, A.M.; Crubézy, E.; Ludes, B.; Drouet, C. Revisiting carbonate quantification in apatite (bio)minerals: A validated FTIR methodology. J. Archaeol. Sci. 2014, 49, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Mysiukiewicz, O.; Barczewski, M. Crystallization of polylactide-based green composites filled with oil-rich waste fillers. J. Polym. Res. 2020, 27, 374. [Google Scholar] [CrossRef]
- Deb, P.; Deoghare, A.B. Effect of Acid, Alkali and Alkali–Acid Treatment on Physicochemical and Bioactive Properties of Hydroxyapatite Derived from Catla catla Fish Scales. Arab. J. Sci. Eng. 2019, 44, 7479–7490. [Google Scholar] [CrossRef]
- Rouhani, P.; Taghavinia, N.; Rouhani, S. Rapid growth of hydroxyapatite nanoparticles using ultrasonic irradiation. Ultrason. Sonochem. 2010, 17, 853–856. [Google Scholar] [CrossRef]
- Karim, B.; LaKrat, M.; Elansari, L.L.; Mejdoubi, E. Synthesis of B-type carbonated hydroxyapatite by a new dissolution-precipitation method. Mater. Today Proc. 2020, 31, S83–S88. [Google Scholar]
- Prasad, A.; Mohan Bhasney, S.; Sankar, M.R.; Katiyar, V. Fish Scale Derived Hydroxyapatite Reinforced Poly (Lactic acid) Polymeric Bio-films: Possibilities for Sealing/locking the Internal Fixation Devices. Mater. Today Proc. 2017, 4, 1340–1349. [Google Scholar] [CrossRef]
- Deb, P.; Deoghare, A.B. Effect of pretreatment processes on physicochemical properties of hydroxyapatite synthesized from Puntius conchonius fish scales. Bull. Mater. Sci. 2019, 42, 3. [Google Scholar]
- Gergely, G.; Wéber, F.; Lukács, I.; Tóth, A.L.; Horváth, Z.E.; Mihály, J.; Balázsi, C. Preparation and characterization of hydroxyapatite from eggshell. Ceram. Int. 2010, 36, 803–806. [Google Scholar] [CrossRef]
- Gopalu, K.; Cho, E.-B.; Thirumurugan, K.; Govindan, S.; Kumar, G.; Kolesnikov, E.; Selvakumar, R. Mesoporous Mn-doped hydroxyapatite nanorods obtained via pyridinium chloride enabled microwave-assisted synthesis by utilizing Donax variabilis seashells for implant applications. Mater. Sci. Eng. C 2021, 126, 112170. [Google Scholar] [CrossRef]
- Tariq, U.; Haider, Z.; Chaudhary, K.; Hussain, R.; Ali, J. Calcium to phosphate ratio measurements in calcium phosphates usinglibs. J. Phys. Conf. Ser. 2018, 1027, 012015. [Google Scholar] [CrossRef]
- Samal, S. Effect of shape and size of filler particle on the aggregation and sedimentation behavior of the polymer composite. Powder Technol. 2020, 366, 43–51. [Google Scholar] [CrossRef]
- Raita, M.S.; Iconaru, S.L.; Groza, A.; Cimpeanu, C.; Predoi, G.; Ghegoiu, L.; Predoi, D. Multifunctional Hydroxyapatite Coated with Arthemisia absinthium Composites. Molecules 2020, 25, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhang, T.; Chen, X.; He, Y.; Liang, X. Model construction of micro-pores in shale: A case study of Silurian Longmaxi Formation shale in Dianqianbei area, SW China. Pet. Explor. Dev. 2018, 45, 412–421. [Google Scholar] [CrossRef]
- Muhammad, N.; Gao, Y.; Iqbal, F.; Ahmad, P.; Ge, R.; Nishan, U.; Ullah, Z. Extraction of biocompatible hydroxyapatite from fish scales using novel approach of ionic liquid pretreatment. Sep. Purif. Technol. 2016, 161, 129–135. [Google Scholar] [CrossRef]
- Kamarudin, S.; Luqman Chuah, A.; Aung, M.M.; Ratnam, C.; Jusoh, E. A study of mechanical and morphological properties of PLA based biocomposites prepared with EJO vegetable oil based plasticiser and kenaf fibres. Mater. Res. Express 2018, 5, 085314. [Google Scholar] [CrossRef]
- Boey, J.Y.; Lee, C.K.; Tay, G.S. Factors Affecting Mechanical Properties of Reinforced Bioplastics: A Review. Polymers 2020, 14, 3737. [Google Scholar] [CrossRef]
- Jodeh, S.; Azzaoui, K.; Mejdoubi, E.; Lamhamdi, A.; Hammouti, B.; Akartasse, N.; Abidi, N. Novel Tricomponenets composites Films From Polylactic Acid/ Hydroxyapatite/ Poly- Caprolactone Suitable For Biomedical Applications. J. Mater. Environ. Sci. 2016, 7, 761–769. [Google Scholar]
- Goreke, M.D.; Alakent, B.; Soyer-Uzun, S. Comparative Study on Factors Governing Binding Mechanisms in Polylactic Acid–Hydroxyapatite and Polyethylene–Hydroxyapatite Systems via Molecular Dynamics Simulations. Langmuir 2020, 36, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Rakmae, S.; Lorprayoon, C.; Ekgasit, S.; Suppakarn, N. Influence of Heat-Treated Bovine Bone-Derived Hydroxyapatite on Physical Properties and in vitro Degradation Behavior of Poly (Lactic Acid) Composites. Polym. Plast. Technol. Eng. 2013, 52, 1043–1053. [Google Scholar] [CrossRef]
- Li, J.; Lu, X.; Zheng, Y. Effect of surface modified hydroxyapatite on the tensile property improvement of HA/PLA composite. Appl. Surf. Sci. 2008, 255, 494–497. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Poly (lactic acid)-based biomaterials: Synthesis, modification and applications. Biomed.Sci. Eng. Technol. 2012, 11, 247–282. [Google Scholar]
- Pandele, A.; Constantinescu, A.; Radu, C.; Miculescu, F.; Voicu, Ş.I.; Ciocan, L. Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films. Materials 2020, 13, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamarudin, S.H.; Abdullah, L.C.; Aung, M.M.; Ratnam, C.T. Thermal and Structural Analysis of Epoxidized Jatropha Oil and Alkaline Treated Kenaf Fiber Reinforced Poly(Lactic Acid) Biocomposites. Polymers 2020, 12, 2604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, Y.; Qian, X.; Hui, D.; Sheng, K. Pyrolysis kinetics and mechanical properties of poly(lactic acid)/bamboo particle biocomposites: Effect of particle size distribution. Nanotechnol. Rev. 2020, 9, 524–533. [Google Scholar] [CrossRef]
- Chinh, N.T.; Manh, V.Q.; Trung, V.Q.; Trang, T.D.M.; Hoang, T. Extraction of hydroxyapatite and collagen from the Vietnamese tilapia scales. Vietnam J. Chem. 2019, 57, 225–228. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Y.; Liu, R.; Tian, C. Preparation and characterization of a novel polylactic acid/hydroxyapatite composite scaffold with biomimetic micro-nanofibrous porous structure. J. Mater. Sci. Mater. Med. 2020, 31, 74. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Heim, H.P.; Feldmann, M. Impact modified PLA-hydroxyapatite composites—Thermo-mechanical properties. Compos. A Appl. Sci. Manuf. 2018, 107, 326–333. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]













| Properties | Materials | |
|---|---|---|
| 1DP-nHAp | 2DP-nHAp | |
| Crystallinity (%) | 71.41 | 80.99 |
| Crystallite size, (nm) | 19.41 | 13.87 |
| BET surface area (cm3/g) | 50 | 41 |
| Total pore volume (cm3/g) | 0.26 | 0.17 |
| Mean pore diameter (nm) | 21.25 | 16.32 |
| Designation | nHAp Content (phr) | Young’s Modulus (GPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| PLA | − | 1.73 ± 0.18 | 38.21 ± 0.95 | 23.39 ± 1.97 |
| 2.5 | 1.94 ± 0.27 | 54.45 ± 1.42 | 14.74 ± 2.92 | |
| 5 | 2.65 ± 0.05 | 66.41 ± 3.63 | 4.32 ± 0.34 | |
| 5 | 2.38 ± 0.11 | 52.21 ± 4.67 | 3.44 ± 0.66 | |
| 10 | 2.02 ± 0.18 | 45.80 ± 1.78 | 4.72 ± 0.59 |
| Designation | Tg (°C) | Tcc (°C) | ∆Hcc (J·g−1) | Tm (°C) | ∆Hm (J·g−1) | Xc (%) |
|---|---|---|---|---|---|---|
| PLA | 60.10 | 127.89 | 3.57 | 151.60 | 3.16 | 7.23 |
| 60.81 | 128.39 | 6.71 | 151.59 | 6.51 | 14.50 | |
| 61.00 | 122.40 | 12.98 | 150.61 | 15.69 | 32.38 | |
| 60.77 | 121.90 | 19.72 | 150.44 | 16.74 | 41.17 | |
| 61.14 | 126.07 | 11.22 | 151.28 | 10.21 | 25.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Injorhor, P.; Trongsatitkul, T.; Wittayakun, J.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Nano-Hydroxyapatite from White Seabass Scales as a Bio-Filler in Polylactic Acid Biocomposite: Preparation and Characterization. Polymers 2022, 14, 4158. https://doi.org/10.3390/polym14194158
Injorhor P, Trongsatitkul T, Wittayakun J, Ruksakulpiwat C, Ruksakulpiwat Y. Nano-Hydroxyapatite from White Seabass Scales as a Bio-Filler in Polylactic Acid Biocomposite: Preparation and Characterization. Polymers. 2022; 14(19):4158. https://doi.org/10.3390/polym14194158
Chicago/Turabian StyleInjorhor, Preeyaporn, Tatiya Trongsatitkul, Jatuporn Wittayakun, Chaiwat Ruksakulpiwat, and Yupaporn Ruksakulpiwat. 2022. "Nano-Hydroxyapatite from White Seabass Scales as a Bio-Filler in Polylactic Acid Biocomposite: Preparation and Characterization" Polymers 14, no. 19: 4158. https://doi.org/10.3390/polym14194158