Cold Plasma-Based Fabrication and Characterization of Active Films Containing Different Types of Myristica fragrans Essential Oil Emulsion
Abstract
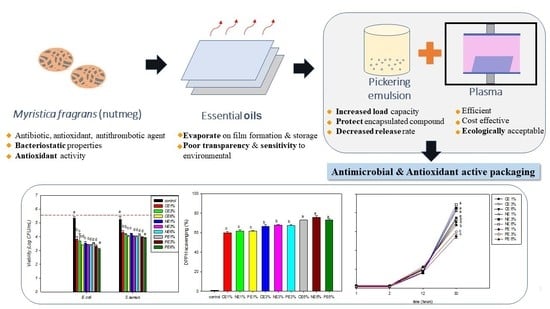
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Emulsion
2.3. Preparation of LDPE-Treated Film
2.4. Optical Properties
2.5. Physical and Mechanical Properties
2.6. Antioxidant Properties
2.7. Antibacterial Assay
2.8. Release Properties
2.9. Statistical Analysis
3. Results and Discussions
3.1. Emulsion Properties
3.2. Optical Properties
3.3. Physical and Mechanical Properties
3.4. Antioxidant Properties
3.5. Anti-Bacterial Assay
3.6. Release Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghadetaj, A.; Almasi, H.; Mehryar, L. Development and characterization of whey protein isolate active films containing nanoemulsions of Grammosciadium ptrocarpum Bioss. essential oil. Food Packag. Shelf Life 2018, 16, 31–40. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Chen, Z.; Wang, T.; Lu, Z.; Hu, W.; Wang, L. Zein/gum Arabic nanoparticle-stabilized Pickering emulsion with thymol as an antibacterial delivery system. Carbohydr. Polym. 2018, 200, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Farahnaky, A.; Javidnia, K.; Majzoobi, M. Comparison of ohmic-assisted hydrodistillation with traditional hydrodistillation for the extraction of essential oils from Thymus vulgaris L. Innov. Food Sci Emerg. Technol. 2012, 14, 85–91. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- D’Souza, S.P.; Chavannavar, S.V.; Kanchanashri, B.; Niveditha, S.B. Pharmaceutical Perspectives of Spices and Condiments as Alternative Antimicrobial Remedy. J. Evid.-Based Complement. Altern. Med. 2017, 22, 1002–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matulyte, I.; Marksa, M.; Ivanauskas, L.; Kalvenien, Z.; Lazauskas, R.; Bernatoniene, J. GC-MS analysis of the composition of the extracts and essential Oil from Myristica fragrans Seeds Using Magnesium Aluminometasilicate as Excipient. Molecules 2019, 24, 1062. [Google Scholar] [CrossRef] [Green Version]
- El-Alfy, A.T.; Abourashed, E.A.; Patel, C.; Mazhari, N.; An, H.R.; Jeon, A. Phenolic compounds from nutmeg (Myristica fragrans Houtt.) inhibit the endocannabinoid-modulating enzyme fatty acid amide hydrolase. J. Pharm. Pharmacol. 2019, 71, 1879–1889. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Ramakrishna, S.; Esmaeili, H.; Bahrani, S.; Koosha, M.; Babapoor, A. Green synthesis of supermagnetic Fe3O4–MgO nanoparticles via Nutmeg essential oil toward superior anti-bacterial and anti-fungal performance. J. Drug Deliv. Sci. Technol. 2019, 54, 101352. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Sivaji, I.; Kandasamy, S.; Duraisamy, S.; Kumar, N.S.; Gurusubramanian, G. Biosynthesis of silver nanoparticles using Myristica fragrans seed (nutmeg) extract and its antibacterial activity against multidrug-resistant (MDR) Salmonella enterica serovar Typhi isolates. Environ. Sci. Pollut. Res. 2017, 24, 14758–14769. [Google Scholar] [CrossRef]
- Galeano, L.Y.; Torres, V.O.; García, S.Á. Evaluation of nutmeg (Myristica fragrans Houtt) as active component during storage of bovine loins. Rev. Cienc. Agrícolas 2018, 35, 48–57. [Google Scholar] [CrossRef]
- Liu, Q.R.; Wang, W.; Qi, J.; Huang, Q.; Xiao, J. Oregano essential oil loaded soybean polysaccharide films: Effect of Pickering type immobilization on physical and antimicrobial properties. Food Hydrocoll. 2019, 87, 165–172. [Google Scholar] [CrossRef]
- Burgos, N.; Mellinas, A.C.; García-serna, E. Nanoencapsulation of Flavor and Aromas in Food Packaging. In Food Packaging; Elsevier Inc.: Philadelphia, PA, USA, 2017; pp. 567–601. [Google Scholar]
- Shi, W.J.; Tang, C.H.; Yin, S.W.; Yin, Y.; Yang, X.Q.; Wu, L.Y.; Zhao, Z.G. Development and characterization of novel chitosan emulsion films via pickering emulsions incorporation approach. Food Hydrocoll. 2016, 52, 253–264. [Google Scholar] [CrossRef]
- Cossu, A.; Wang, M.S.; Chaudhari, A.; Nitin, N. Antifungal activity against Candida albicans of starch Pickering emulsion with thymol or amphotericin B in suspension and calcium alginate films. Int. J. Pharm. 2015, 493, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnia, M.; Khaledabad, M.A.; Almasi, H. Optimization of Ziziphora clinopodiodes essential oil microencapsulation by whey protein isolate and pectin: A comparative study. Int. J. Biol. Macromol. 2017, 101, 958–966. [Google Scholar] [CrossRef]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Gavahian, M.; Meng-Jen, T.; Khaneghah, A.M. Emerging techniques in food science: The resistance of chlorpyrifos pesticide pollution against arc and dielectric barrier discharge plasma. Qual. Assur. Saf. Crops Foods 2020, 12, 9–17. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljević, V.; O’Donnell, C.P.; Bourke, P.; Keener, K.M.; Cullen, P.J. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Martins, H.H.A.; Manrich, A.; Otoni, C.G.; Carvalho, E.E.N.; Piccoli, R.H.; Oliveira, J.E.; Pinheiro, A.C.M.; Mattoso, L.H.C. Correlating emulsion characteristics with the properties of active starch films loaded with lemongrass essential oil. Food Hydrocoll. 2020, 100, 105428. [Google Scholar] [CrossRef]
- González, A.; Gastelú, G.; Barrera, G.N.; Ribotta, P.D.; Álvarez Igarzabal, C.I. Preparation and characterization of soy protein films reinforced with cellulose nanofibers obtained from soybean by-products. Food Hydrocoll. 2019, 89, 758–764. [Google Scholar] [CrossRef]
- Hu, Y.; Shi, L.; Ren, Z.; Hao, G.; Chen, J.; Weng, W. Characterization of emulsion films prepared from soy protein isolate at different preheating temperatures. J. Food Eng. 2021, 309, 110697. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Tian, B.; Li, D.; Liu, C.; Jiang, B.; Feng, Z. Preparation and characterization of coating based on protein nanofibers and polyphenol and application for salted duck egg yolks. Foods 2020, 9, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Wang, L.; Zhu, M.; Wu, S.; Wang, X.; Li, D.; Liu, C.; Feng, Z.; Tian, C. Separation, structural characteristics and biological activity of lactic acid bacteria exopolysaccharides separated by aqueous two-phase system. LWT 2021, 147, 111617. [Google Scholar] [CrossRef]
- Tian, B.; Cheng, J.; Zhang, T.; Liu, Y.; Chen, D. Multifunctional chitosan-based film loaded with hops β-acids: Preparation, characterization, controlled release and antibacterial mechanism. Food Hydrocoll. 2022, 124, 107337. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Wong, L.W.; Loke, X.J.; Chang, C.K.; Ko, W.C.; Hou, C.Y.; Hsieh, C.W. Use of the plasma-treated and chitosan/gallic acid-coated polyethylene film for the preservation of tilapia (Orechromis niloticus) fillets. Food Chem. 2020, 329, 126989. [Google Scholar] [CrossRef]
- Loke, X.J.; Chang, C.K.; Hou, C.Y.; Cheng, K.C.; Hsieh, C.W. Plasma-treated polyethylene coated with polysaccharide and protein containing cinnamaldehyde for active packaging films and applications on tilapia (Orechromis niloticus) fillet preservation. Food Control 2021, 125, 1–10. [Google Scholar] [CrossRef]
- Han, H.S.; Song, K.B. Noni (Morinda citrifolia) fruit polysaccharide films containing blueberry (Vaccinium corymbosum) leaf extract as an antioxidant packaging material. Food Hydrocoll. 2021, 112, 1–8. [Google Scholar] [CrossRef]
- Grzegorzewski, F.; Rohn, S.; Kroh, L.W.; Geyer, M.; Schlüter, O. Surface morphology and chemical composition of lamb’s lettuce (Valerianella locusta) after exposure to a low-pressure oxygen plasma. Food Chem. 2010, 122, 1145–1152. [Google Scholar] [CrossRef]
- Theapsak, S.; Watthanaphanit, A.; Rujiravanit, R. Preparation of chitosan-coated polyethylene packaging films by DBD plasma treatment. ACS Appl. Mater. Interfaces 2012, 4, 2474–2482. [Google Scholar] [CrossRef]
- Dammak, I.; de Carvalho, R.A.; Trindade, C.S.F.; Lourenço, R.V.; do Amaral, S.P.J. Properties of active gelatin films incorporated with rutin-loaded nanoemulsions. Int. J. Biol. Macromol. 2017, 98, 39–49. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Wang, X.; Dong, S.; Sun, Y.; Zhao, Z. The properties of chitosan/zein blend film and effect of film on quality of mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 155, 47–56. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1985, 181, 1199–1200. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Nurjanah, N.; Jacoeb, A.M.; Asmara, D.A.; Hidayat, T. Phenol Component of Fresh and Boiled Sea Grapes (Caulerpa sp.) From Tual, Maluku. Food Sci. J. 2019, 1, 31. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Yang, J.; Xue, J. Characterization of antimicrobial poly (lactic acid)/poly(trimethylene carbonate) films with cinnamaldehyde. J. Mater. Sci. 2015, 50, 1150. [Google Scholar] [CrossRef]
- Shokri, S.; Parastouei, K.; Taghdir, M.; Abbaszadeh, S. Application an edible active coating based on chitosan- Ferulago angulata essential oil nanoemulsion to shelf life extension of Rainbow trout fillets stored at 4 °C. Int. J. Biol. Macromol. 2020, 153, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Söğüt, E. Properties of Solvent Cast Polycaprolactone Films Containing Pomegranate Seed Oil Stabilized with Nanocellulose. Turk. J. Agric.-Food Sci. Technol. 2019, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Sun, Z.; Liu, Z.; Yang, Y.; Liu, Z.; Wang, Y.; Jiang, B.; Feng, Z.; Liu, C. High efficiency desalination of wasted salted duck egg white and processing into food-grade pickering emulsion stabilizer. LWT 2022, 161, 113337. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, Q.; Wang, L.; Zhang, Y.; Jiang, B.; Li, D.; Feng, Z.; Liu, C. Preparation and evaluation of a novel high internal phase Pickering emulsion based on whey protein isolate nanofibrils derived by hydrothermal method. Food Hydrocoll. 2022, 123, 107180. [Google Scholar] [CrossRef]
- Galus, S. Functional properties of soy protein isolate edible films as affected by rapeseed oil concentration. Food Hydrocoll. 2018, 85, 233–241. [Google Scholar] [CrossRef]
- Dong, S.; Gao, A.; Zhao, Y.; Li, Y.T.; Chen, Y. Characterization of physicochemical and structural properties of atmospheric cold plasma (ACP) modified zein. Food Bioprod. Process. 2017, 106, 65–74. [Google Scholar] [CrossRef]
- Wong, L.W.; Hou, C.Y.; Hsieh, C.C.; Chang, C.K.; Wu, Y.S.; Hsieh, C.W. Preparation of antimicrobial active packaging film by capacitively coupled plasma treatment. LWT 2020, 117, 108612. [Google Scholar] [CrossRef]
- Almasi, H.; Zandi, M.; Beigzadeh, S.; Haghju, S.; Mehrnow, N. Chitosan films incorporated with nettle (Urtica dioica L.) extract-loaded nanoliposomes: II. Antioxidant activity and release properties. J. Microencapsul. 2016, 33, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Fasihi, H.; Fazilati, M.; Hashemi, M.; Noshirvani, N. Novel carboxymethyl cellulose-polyvinyl alcohol blend films stabilized by Pickering emulsion incorporation method. Carbohydr. Polym. 2017, 167, 79–89. [Google Scholar] [CrossRef]
- Li, S. Effect of Pickering emulsion on the mechanical performances and fracture toughness of epoxy composites. Polym. Adv. Technol. 2020, 31, 722–730. [Google Scholar] [CrossRef]
- Wardana, A.A.; Koga, A.; Tanaka, F.; Tanaka, F. Antifungal features and properties of chitosan/sandalwood oil Pickering emulsion coating stabilized by appropriate cellulose nanofiber dosage for fresh fruit application. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Muller, J.; González-Martínez, C.; Chiralt, A. Poly(lactic) acid (PLA) and starch bilayer films, containing cinnamaldehyde, obtained by compression moulding. Eur. Polym. J. 2017, 95, 56–70. [Google Scholar] [CrossRef]
- Bonilla, J.; Talón, E.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of the incorporation of antioxidants on physicochemical and antioxidant properties of wheat starch-chitosan films. J. Food Eng. 2013, 118, 271–278. [Google Scholar] [CrossRef]
- Silva Damasceno, E.T.; Almeida, R.R.; de Carvalho, S.Y.B.; de Carvalho, G.S.G.; Mano, V.; Pereira, A.C.; Guimaraes, L.G. Lippia origanoides Kunth. essential oil loaded in nanogel based on the chitosan and ρ-coumaric acid: Encapsulation efficiency and antioxidant activity. Ind. Crops Prod. 2018, 125, 85–94. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. β-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf. B Biointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A.; Kremensas, A. Nutmeg filler as a natural compound for the production of polyurethane composite foams with antibacterial and anti-aging properties. Polym. Test. 2020, 86, 1–13. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Leontiou, A.; Siasou, Z.; Karakassides, M.A. Development of Poly (L-Lactic Acid)/Chitosan/Basil Oil Active Packaging Films via a Melt-Extrusion Process Using Novel Chitosan/Basil Oil Blends. Processes 2021, 9, 88. [Google Scholar] [CrossRef]
- Ginting, B.; Maira, R.M.; Helwati, H.; Desiyana, L.S.; Mujahid, R. Isolation of essensial oil of nutmeg (Myristica fragrans Houtt) and antioxidant activity test with DPPH. J. Nat. 2018, 18, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Dammak, I.; Lourenço, R.V.; Sobral, P.J.A. Active gelatin films incorporated with Pickering emulsions encapsulating hesperidin: Preparation and physicochemical characterization. J. Food Eng. 2019, 240, 9–20. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zu, Y.G.; Fu, Y.J. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mcclements, D.J.; Mclandsborough, L.A. Interaction between emulsion droplets and Escherichia coli cells. J. Food Sci. 2001, 66, 570–657. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]







| Emulsion | Droplet Size (nm) | Zeta Potential (mV) | Polydispersity Index |
|---|---|---|---|
| CE 1% | 1533.15 ± 0.31 a | 40.80 ± 0.25 a | 0.66 ± 0.37 a |
| CE 3% | 1615.70 ± 0.13 a | 38.38 ± 0.29 b | 0.81 ± 0.32 a |
| CE 6% | 1731.23 ± 0.24 a | 26.54 ± 0.58 c | 0.93 ± 0.11 a |
| NE 1% | 170.93 ± 0.11 b | 8.47 ± 0.26 d | 0.22 ± 0.01 c |
| NE 3% | 197.46 ± 0.52 b | 12.10 ± 0.01 c,d | 0.38 ± 0.02 b |
| NE 6% | 155.60 ± 0.10 b | 11.13 ± 0.03 c,d | 0.25 ± 0.02 c |
| PE 1% | 244.76 ± 0.86 b | 21.30 ± 0.26 c | 0.39 ± 0.01 b |
| PE 3% | 236.98 ± 0.07 b | 18.34 ± 0.10 c,d | 0.29 ± 0.04 c |
| PE 6% | 233.56 ± 0.11 b | 19.43 ± 0.08 c,d | 0.32 ± 0.04 b,c |
| Emulsion | L | a | b | ΔE | YI | Opacity | WCA |
|---|---|---|---|---|---|---|---|
| Control | 93.01 ± 0.10 a | 0.10 ± 0.16 a | −6.76 ± 0.16 d | - | −10.38 ± 0.16 e | 0.45 ± 0.16 f | 4.88 ± 0.01 e |
| CE 1% | 27.79 ± 0.16 b,c | −11.80 ± 0.19 e | 2.35 ± 0.18 c | 1.70 ± 0.16 | 12.10 ± 0.18 d | 1.05 ± 0.04 e | 6.22 ± 0.01 d |
| CE 3% | 24.76 ± 0.55 c | −10.09 ± 0.09 c | 2.88 ± 0.88 c | 0.08 ± 0.88 | 16.40 ± 0.49 c | 1.07 ± 0.05 e | 7.63 ± 0.01 b |
| CE 6% | 25.20 ± 0.35 c | −9.47 ± 0.15 b | 3.15 ± 0.61 c | 0.35 ± 0.31 | 17.89 ± 0.35 b,c | 1.06 ± 0.15 e | 7.73 ± 0.01 b |
| NE 1% | 25.20 ± 0.13 c | −10.78 ± 0.18 d | 4.22 ± 0.29 b | 0.31 ± 0.04 | 23.92 ± 0.08 b | 1.10 ± 0.05 d | 4.94 ± 0.05 d,e |
| NE 3% | 31.21 ± 0.65 b | −11.01 ± 0.50 d | 6.58 ± 0.40 a | 0.49 ± 0.71 | 30.10 ± 0.66 a | 1.15 ± 0.05 c | 5.72 ± 0.10 d |
| NE 6% | 30.95 ± 0.21 b | −12.18 ± 0.07 e | 6.77 ± 0.10 a | 0.36 ± 0.21 | 31.24 ± 0.21 a | 1.15 ± 0.06 c | 8.83 ± 0.09 a |
| PE 1% | 27.05 ± 0.55 b,c | −13.70 ± 0.55 g | 5.91 ± 0.14 a | 0.50 ± 0.22 | 21.55 ± 0.60 b | 1.46 ± 0.47 b | 4.66 ± 0.05 e |
| PE 3% | 26.72 ± 0.49 b,c | −12.86 ± 0.33 f | 4.19 ± 0.69 b | 0.40 ± 0.43 | 22.57 ± 0.55 b | 1.44 ± 0.10 b | 6.62 ± 0.01 c |
| PE 6% | 31.24 ± 0.02 b | −11.68 ± 0.12 e | 4.71 ± 0.54 b | 0.40 ± 0.03 | 31.42 ± 0.04 a | 2.34 ± 0.05 a | 8.28 ± 0.07 a |
| Emulsion | SS (Mpa) | TS (Mpa) | Thickness | WVP | OP |
|---|---|---|---|---|---|
| (mm) | (×10−7 g·m−1·s−1·Pa−1) | (×10−12 g·m·m−2·s−1·Pa−1) | |||
| Control | 20.0 ± 0.26 a | 18.3 ± 0.25 a | 0.034 ± 0.03 b | 2.45 ± 0.01 a | 6.16 ± 0.01 a |
| CE 1% | 15.5 ± 0.26 d | 18.6 ± 0.34 a | 0.034 ± 0.01 b | 1.47 ± 0.01 b | 4.50 ± 0.01 b |
| CE 3% | 15.5 ± 0.28 d | 12.3 ± 0.25 d | 0.034 ± 0.00 b | 1.41 ± 0.06 c | 4.37 ± 0.01 b |
| CE 6% | 14.5 ± 0.28 e | 11.5 ± 0.28 d | 0.034 ± 0.10 b | 1.39 ± 0.01 c | 4.42 ± 0.01 b |
| NE 1% | 14.3 ± 0.01 e | 11.3 ± 0.01 d | 0.034 ± 0.01 b | 1.46 ± 0.01 b | 4.98 ± 0.01 b |
| NE 3% | 14.9 ± 0.26 d | 11.8 ± 0.01 d | 0.035 ± 0.00 b | 1.45 ± 0.01 b | 3.18 ± 0.01 c |
| NE 6% | 15.1 ± 0.05 d | 18.9 ± 0.05 a | 0.038 ± 0.02 a | 1.20 ± 0.01 c | 2.37 ± 0.01 d |
| PE 1% | 17.0 ± 0.28 c | 13.4 ± 0.12 c | 0.035 ± 0.01 b | 1.46 ± 0.01 b | 3.32 ± 0.01 c |
| PE 3% | 19.4 ± 0.23 b | 15.3 ± 0.01 b | 0.039 ± 0.11 a | 1.52 ± 0.09 b | 3.04 ± 0.01 c |
| PE 6% | 23.3 ± 0.40 a | 18.4 ± 0.02 a | 0.039 ± 0.01 a | 1.59 ± 0.20 b | 3.05 ± 0.01 c |
| Emulsion | Higuchi | Korsmeyer–Peppas | |||
|---|---|---|---|---|---|
| K1 | R2 | K2 | n | R2 | |
| CE 1% | 2.5627 | 0.9727 | 1.1136 | 0.1334 | 0.9805 |
| CE 3% | 2.5829 | 0.989 | 1.0464 | 0.2295 | 0.9816 |
| CE 6% | 2.6465 | 0.9843 | 1.0669 | 0.1872 | 0.9721 |
| NE 1% | 2.8933 | 0.9809 | 1.1831 | 0.0707 | 0.9643 |
| NE 3% | 2.8512 | 0.9687 | 1.2052 | 0.0236 | 0.9638 |
| NE 6% | 2.8522 | 0.9844 | 1.0586 | 0.2529 | 0.9659 |
| PE 1% | 3.1184 | 0.9894 | 1.0212 | 0.0348 | 0.9968 |
| PE 3% | 2.9334 | 0.9862 | 1.1089 | 0.2674 | 0.9859 |
| PE 6% | 2.49 | 0.9912 | 1.0225 | 0.4515 | 0.9746 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yudhistira, B.; Sulaimana, A.S.; Punthi, F.; Chang, C.-K.; Lung, C.-T.; Santoso, S.P.; Gavahian, M.; Hsieh, C.-W. Cold Plasma-Based Fabrication and Characterization of Active Films Containing Different Types of Myristica fragrans Essential Oil Emulsion. Polymers 2022, 14, 1618. https://doi.org/10.3390/polym14081618
Yudhistira B, Sulaimana AS, Punthi F, Chang C-K, Lung C-T, Santoso SP, Gavahian M, Hsieh C-W. Cold Plasma-Based Fabrication and Characterization of Active Films Containing Different Types of Myristica fragrans Essential Oil Emulsion. Polymers. 2022; 14(8):1618. https://doi.org/10.3390/polym14081618
Chicago/Turabian StyleYudhistira, Bara, Andi Syahrullah Sulaimana, Fuangfah Punthi, Chao-Kai Chang, Chun-Ta Lung, Shella Permatasari Santoso, Mohsen Gavahian, and Chang-Wei Hsieh. 2022. "Cold Plasma-Based Fabrication and Characterization of Active Films Containing Different Types of Myristica fragrans Essential Oil Emulsion" Polymers 14, no. 8: 1618. https://doi.org/10.3390/polym14081618