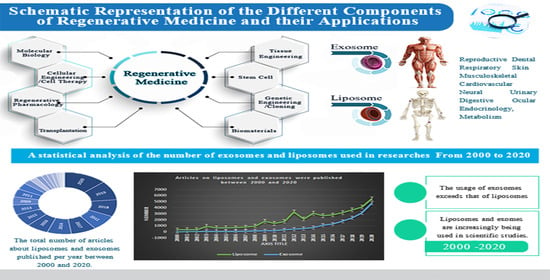
A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering
Abstract
:1. Introduction
1.1. The Current State and Background of Tissue Engineering and Regenerative Medicine
1.2. Biology of Exosomes and Liposomes
1.2.1. Liposomes
Major Structural Components of Liposomes
Phospholipids
Cholesterol
Classification of Liposomes
1.2.2. Exosomes
The Discovery of Exosomes
Mechanisms of Exosome Formation and Biogenesis
Composition of Exosomes
1.2.3. The Exosomes and Liposomes Similarities and Differences
1.2.4. Engineered Extracellular Vesicles by Way of Membrane Fusion with Synthetic Lipids
1.2.5. Scaffold Techniques and Designs Based on Liposome and Exosome in Tissue Engineering
1.2.6. Combining Scaffolds with Liposomes
1.2.7. Combining Scaffolds with Exosomes
2. The Application of Musculoskeletal Tissue Engineering Scaffolds Modified with Liposomes or Exosomes
2.1. Regeneration of Bone with Exosomes
2.2. Regeneration of Bones with Liposomes
2.3. Cartilage Regeneration with Exosomes
2.4. Treating Osteoporosis with Exosomes
3. The Application of Craniomaxillofacial Tissue Engineering Scaffolds Modified with Liposomes or Exosomes
Craniofacial Bone Regeneration with Exosomes
4. The Application of Skin Tissue Engineering Scaffolds Modified with Liposomes or Exosomes
Treatment Diseases Associated with the Skin with Liposomes
5. The Application of Neural Tissue Engineering Scaffolds Modified with Liposomes or Exosomes
5.1. Reparation of the Spinal Cord with Liposome
5.2. Spinal Cord Injury Treatment with Exosomes
5.3. Exosomes as a Traumatic Brain Injury (TBI) Treatment
5.4. Therapy for Head Injuries with Exosomes
6. The Application of Dental Tissue Engineering Scaffolds Modified with Liposomes or Exosomes
Regeneration of Teeth
7. The Application of Scaffolds for Reproductive System Modified with Liposomes or Exosomes
Female Fertility Preservation with Liposomes
8. The Usage of Scaffolds Composed with Liposomes in Sickness
8.1. Breast Cancer
8.2. Cancer Treatment
8.3. Diabetes Mellitus
8.4. Inflammatory Disorders
8.5. The Human Immunodeficiency Viruses (HIV)
8.6. Anti-Bacterial Activities and Applications
8.7. Healing of Acute Wounds
9. The Usage of Scaffolds Composed with Exosomes in Sickness
9.1. Wound Healing
9.2. Engineering Smart Exosome–Liposome Hybrid
10. Critical Discussion and Perspective
11. Closing Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Della Bona, A.; Cavalcanti, B.; Nör, J.E. Tissue engineering: From research to dental clinics. Dent. Mater. 2012, 28, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Langer, R.; Vacanti, J. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Ramos, T.A.D.S.; Moroni, L. Tissue Engineering and Regenerative Medicine 2019: The Role of Biofabrication—A Year in Review. Tissue Eng. Part C Methods 2020, 26, 91–106. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef]
- Hsu, M.-N.; Chang, Y.-H.; Truong, V.A.; Lai, P.-L.; Nguyen, T.K.N.; Hu, Y.-C. CRISPR technologies for stem cell engineering and regenerative medicine. Biotechnol. Adv. 2019, 37, 107447. [Google Scholar] [CrossRef]
- Pulgarin, D.A.V. CRISPR/Cas Systems in Tissue Engineering: A Succinct Overview of Current Use and Future Opportunities. Curr. Trends Biomed. Eng. Biosci. 2017, 5. [Google Scholar] [CrossRef]
- Samsudin, R. Stem Cell and Tissue Engineering—The Challenge of Imitating Nature. Malays. J. Med. Sci. 2003, 10, 1–3. [Google Scholar]
- Vishwakarma, A.; Sharpe, P.; Shi, S.; Ramalingam, M. Chapter 1—An Introduction to Stem Cell Biology and Tissue Engineering. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Academic Press: Cambridge, MA, USA, 2015; pp. 1–13. [Google Scholar]
- Hirschi, K.K.; Li, S.; Roy, K. Induced Pluripotent Stem Cells for Regenerative Medicine. Annu. Rev. Biomed. Eng. 2014, 16, 277–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [Green Version]
- Shajkumar, A. Chapter 17—Future of Nanotechnology in Tissue Engineering. In Nanotechnology Applications for Tissue Engineering; Thomas, S., Grohens, Y., Ninan, N., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 289–306. [Google Scholar]
- Stratakis, E. Novel Biomaterials for Tissue Engineering 2018. Int. J. Mol. Sci. 2018, 19, 3960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keane, T.J.; Badylak, S.F. Biomaterials for tissue engineering applications. Semin. Pediatr. Surg. 2014, 23, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.W. The history of prosthetic dentistry. J. Prosthet. Dent. 1959, 9, 841–846. [Google Scholar] [CrossRef]
- Abraham, C.M. A Brief Historical Perspective on Dental Implants, Their Surface Coatings and Treatments. Open Dent. J. 2014, 8, 50–55. [Google Scholar] [CrossRef]
- Nerlich, A.G.; Zink, A.; Szeimies, U.; Hagedorn, H.G. Ancient Egyptian prosthesis of the big toe. Lancet 2000, 356, 2176–2179. [Google Scholar] [CrossRef]
- Zimbler, M.S. Gaspare Tagliacozzi (1545–1599): Renaissance surgeon. Arch. Facial. Plast. Surg. 2001, 3, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, J. Tissue engineering and regenerative medicine: From first principles to state of the art. J. Pediatr. Surg. 2010, 45, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.P. Development of tissue bank. Indian J. Plast. Surg. 2012, 45, 396–402. [Google Scholar] [CrossRef]
- Webster, J.P. Refrigerated Skin Grafts. Ann. Surg. 1944, 120, 431–448. [Google Scholar] [CrossRef]
- Polge, C.; Smith, A.U.; Parkes, A.S. Revival of Spermatozoa after Vitrification and Dehydration at Low Temperatures. Nature 1949, 164, 666. [Google Scholar] [CrossRef]
- Harrison, J.H.; Merrill, J.P.; Murray, J.E. Renal homotransplantation in identical twins. Surg. Forum 1956, 6, 432–436. [Google Scholar] [PubMed]
- Starzl, T.E.; Barker, C. The origin of clinical organ transplantation revisited. JAMA 2009, 301, 2041–2043. [Google Scholar] [CrossRef] [Green Version]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Q.; Du, Y. Advances in the Application of Biomimetic Endometrium Interfaces for Uterine Bioengineering in Female Infertility. Front. Bioeng. Biotechnol. 2020, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Louis, F.; Matsusaki, M. 15—Adipose tissue engineering. In Biomaterials for Organ and Tissue Regeneration; Vrana, N.E., Knopf-Marques, H., Barthes, J., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 393–423. [Google Scholar]
- Murphy, C.; Liaw, L.; Reagan, M.R. In vitro tissue-engineered adipose constructs for modeling disease. BMC Biomed. Eng. 2019, 1, 1–19. [Google Scholar] [CrossRef]
- Zhao, Y.; Eng, G.; Lee, B.; Radisic, M.; Novakovic, G. Chapter 32—Cardiac tissue engineering. In Principles of Tissue Engineering, 5th ed.; Lanza, R., Langer, R., Vacanti, J., Atala, A., Eds.; Academic Press: Boston, MA, USA, 2020; pp. 593–616. [Google Scholar]
- Hsia, K.; Yao, C.-L.; Chen, W.-M.; Chen, J.-H.; Lee, H.; Lu, J.-H. Scaffolds and Cell-Based Tissue Engineering for Blood Vessel Therapy. Cells Tissues Organs 2016, 202, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zi-Ying, Y.; Bian, G.-L.; Huang, H.-Y.; Shen, H.; Yang, J.-J.; Yang, Z.-Y.; Shen, Z.-Y. The combination of stem cells and tissue engineering: An advanced strategy for blood vessels regeneration and vascular disease treatment. Stem Cell Res. Ther. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Doi, R.; Obata, T.; Hatachi, G.; Nagayasu, T. Lung Microvascular Niche, Repair, and Engineering. Front. Bioeng. Biotechnol. 2020, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, S.E.; Wagner, D.E. Acellular human lung scaffolds to model lung disease and tissue regeneration. Eur. Respir. Rev. 2018, 27, 180021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wragg, N.; Burke, L.; Wilson, S.L. A critical review of current progress in 3D kidney biomanufacturing: Advances, challenges, and recommendations. Ren. Replace. Ther. 2019, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Anandakrishnan, N.; Azeloglu, E.U. Kidney tissue engineering for precision medicine. Nat. Rev. Nephrol. 2020, 16, 623–624. [Google Scholar] [CrossRef]
- Dehkordi, A.N.; Babaheydari, F.M.; Chehelgerdi, M.; Dehkordi, S.R. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.R.; Navarro, J.; Coburn, J.C.; Mahadik, B.; Molnar, J.; Iv, J.H.H.; Nam, A.J.; Fisher, J.P. Current and Future Perspectives on Skin Tissue Engineering: Key Features of Biomedical Research, Translational Assessment, and Clinical Application. Adv. Healthc. Mater. 2019, 8, e1801471. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, H.; Tsunoi, Y.; Akagi, T.; Sato, S.; Akashi, M.; Saitoh, D. A novel strategy to engineer pre-vascularized 3-dimensional skin substitutes to achieve efficient, functional engraftment. Sci. Rep. 2019, 9, 7797. [Google Scholar] [CrossRef] [PubMed]
- Dhasmana, A.; Singh, A.; Rawal, S. Biomedical grafts for tracheal tissue repairing and regeneration “Tracheal tissue engineering: An overview”. J. Tissue Eng. Regen. Med. 2020, 14, 653–672. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Yoon, J.-K.; Lee, J.B.; Shin, Y.M.; Lee, K.-W.; Bae, S.-W.; Lee, J.; Yu, J.; Jung, C.-R.; Youn, Y.-N.; et al. Experimental Tracheal Replacement Using 3-dimensional Bioprinted Artificial Trachea with Autologous Epithelial Cells and Chondrocytes. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Conder, R.K.; Li, V.; Lutolf, M.P.; Vallier, L.; Chan, S.; Grikscheit, T.C.; Jensen, K.B.; De Coppi, P. Tissue-Engineering the Intestine: The Trials before the Trials. Cell Stem Cell 2019, 24, 855–859. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.-K.; Ren, K.; Zuo, J.; Ding, J.; Chen, X. Thermosensitive Hydrogels as Scaffolds for Cartilage Tissue Engineering. Biomacromolecules 2019, 20, 1478–1492. [Google Scholar] [CrossRef]
- Gupta, N.; Cruz, M.A.; Nasser, P.; Rosenberg, J.D.; Iatridis, J.C. Fibrin-Genipin Hydrogel for Cartilage Tissue Engineering in Nasal Reconstruction. Ann. Otol. Rhinol. Laryngol. 2019, 128, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Horst, M.; Eberli, D.; Gobet, R.; Salemi, S. Tissue Engineering in Pediatric Bladder Reconstruction—The Road to Success. Front. Pediatr. 2019, 7, 91. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Aroca, Á.; Vera-Donoso, C.D.; Moreno-Manzano, V. Bioengineering Approaches for Bladder Regeneration. Int. J. Mol. Sci. 2018, 19, 1796. [Google Scholar] [CrossRef] [Green Version]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2018, 13, 58–75. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Nakamura-Yamada, S.; Konoki, R.; Baba, S. Promising advances in clinical trials of dental tissue-derived cell-based regenerative medicine. Stem Cell Res. Ther. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Fernández-Pérez, J.; Masterton, S.; Madden, P.W.; Bhattacharjee, P. Designing Scaffolds for Corneal Regeneration. Adv. Funct. Mater. 2020, 30, 1908996. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Yang, X.; Song, W.; Ren, L. Construction and Evaluation of Collagen-Based Corneal Grafts Using Polycaprolactone To Improve Tension Stress. ACS Omega 2020, 5, 674–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.-X.; Han, N.; Kou, Y.-H.; Zhu, Q.-T.; Liu, X.-L.; Quan, D.-P.; Chen, J.-G.; Jiang, B.-G. Tissue engineering for the repair of peripheral nerve injury. Neural Regen. Res. 2019, 14, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front. Bioeng. Biotechnol. 2019, 7, 337. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Lowe, B.; Hardy, J.G.; Walsh, L. Optimizing Nanohydroxyapatite Nanocomposites for Bone Tissue Engineering. ACS Omega 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, M.J.; Qi, B.; Bayaniahangar, R.; Araban, V.; Bakhtiary, Z.; Doschak, M.R.; Goh, B.C.; Shokouhimehr, M.; Vali, H.; Presley, J.F.; et al. Nanomaterials for bone tissue regeneration: Updates and future perspectives. Nanomedicine 2019, 14, 2987–3006. [Google Scholar]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Liu, L.; Xiang, Y.; Lu, Y.; Deng, L.; Zhang, H.; Santos, H.A.; Cui, W. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials 2020, 232, 119706. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 2014, 11, 20140459. [Google Scholar] [CrossRef] [Green Version]
- Richter, R.P.; Bérat, R.; Brisson, A.R. Formation of Solid-Supported Lipid Bilayers: An Integrated View. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef]
- Lian, T.; Ho, R.J.Y. Trends and Developments in Liposome Drug Delivery Systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, W.; Lamprecht, A. Targeted drug-delivery approaches by nanoparticulate carriers in the therapy of inflammatory diseases. J. R. Soc. Interface 2009, 7, S55–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voinea, M.; Simionescu, M. Designing of ‘intelligent’ liposomes for efficient delivery of drugs. J. Cell. Mol. Med. 2002, 6, 465–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, F.; Zhang, X.; Zhang, H.; Qu, X.; Chen, D.; Servos, M.; Mäkilä, E.; Salonen, J.; Santos, H.A.; Hai, M.; et al. Inhibition of Multidrug Resistance of Cancer Cells by Co-Delivery of DNA Nanostructures and Drugs Using Porous Silicon Nanoparticles@Giant Liposomes. Adv. Funct. Mater. 2015, 25, 3330–3340. [Google Scholar] [CrossRef]
- Gómez-Hens, A.; Romero, J.M.F. The role of liposomes in analytical processes. TrAC Trends Anal. Chem. 2005, 24, 9–19. [Google Scholar] [CrossRef]
- Levchenko, T.S.; Hartner, W.C.; Torchilin, V.P. Liposomes in Diagnosis And Treatment Of Cardiovascular Disorders. Methodist DeBakey Cardiovasc. J. 2012, 8, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Martina, M.-S.; Fortin, J.-P.; Ménager, C.; Clément, O.; Barratt, G.; Grabielle-Madelmont, C.; Gazeau, F.; Cabuil, V.; Lesieur, S. Generation of Superparamagnetic Liposomes Revealed as Highly Efficient MRI Contrast Agents for in Vivo Imaging. J. Am. Chem. Soc. 2005, 127, 10676–10685. [Google Scholar] [CrossRef] [PubMed]
- Boerman, O.; Laverman, P.; Oyen, W.; Corstens, F.; Storm, G. Radiolabeled liposomes for scintigraphic imaging. Prog. Lipid Res. 2000, 39, 461–475. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes in biology and medicine. Adv. Exp. Med. Biol. 2007, 620, 117–128. [Google Scholar] [PubMed] [Green Version]
- Shukla, S.; Haldorai, Y.; Hwang, S.K.; Bajpai, V.K.; Huh, Y.S.; Han, Y.-K. Current Demands for Food-Approved Liposome Nanoparticles in Food and Safety Sector. Front. Microbiol. 2017, 8, 2398. [Google Scholar] [CrossRef] [Green Version]
- Moussaoui, N.; Cansell, M.; Denizot, A. Marinosomes®, marine lipid-based liposomes: Physical characterization and potential application in cosmetics. Int. J. Pharm. 2002, 242, 361–365. [Google Scholar] [CrossRef]
- Xia, S.; Xu, S. Ferrous sulfate liposomes: Preparation, stability and application in fluid milk. Food Res. Int. 2005, 38, 289–296. [Google Scholar] [CrossRef]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Cummings, E.B.; Throckmorton, D.J. Fluorescent Liposome Flow Markers for Microscale Particle-Image Velocimetry. Anal. Chem. 2001, 73, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- An, S.Y.; Bui, M.-P.N.; Nam, Y.J.; Han, K.N.; Li, C.A.; Choo, J.; Lee, E.K.; Katoh, S.; Kumada, Y.; Seong, G.H. Preparation of monodisperse and size-controlled poly(ethylene glycol) hydrogel nanoparticles using liposome templates. J. Colloid Interface Sci. 2009, 331, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Rahnfeld, L.; Luciani, P. Injectable Lipid-Based Depot Formulations: Where Do We Stand? Pharmaceutics 2020, 12, 567. [Google Scholar] [CrossRef]
- Karagoz, B.; Payne, M.; Reinicker, A.; Kondratyuk, P.; Gellman, A.J. A Most Enantioselective Chiral Surface: Tartaric Acid on All Surfaces Vicinal to Cu(110). Langmuir 2019, 35, 16438–16443. [Google Scholar] [CrossRef]
- Sivan, S.; Schroeder, A.; Verberne, G.; Merkher, Y.; Diminsky, D.; Priev, A.; Maroudas, A.; Halperin, G.; Nitzan, D.; Etsion, I.; et al. Liposomes Act as Effective Biolubricants for Friction Reduction in Human Synovial Joints. Langmuir 2010, 26, 1107–1116. [Google Scholar] [CrossRef]
- Kulkarni, M.; Greiser, U.; O’Brien, T.; Pandit, A. Liposomal gene delivery mediated by tissue-engineered scaffolds. Trends Biotechnol. 2010, 28, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Bai, Y.; Pan, J.; Wang, H.; Li, H.; Xu, X.; Fu, X.; Shi, R.; Luo, Z.; Li, Y.; et al. A hybrid 3D-printed aspirin-laden liposome composite scaffold for bone tissue engineering. J. Mater. Chem. B 2019, 7, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R. Liposomes: Applications in Medicine. J. Biomater. Appl. 2001, 16, 3–21. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [Green Version]
- Swaminathan, J.; Ehrhardt, C. Liposomes for Pulmonary Drug Delivery. In Controlled Pulmonary Drug Delivery; Smyth, H.D.C., Hickey, A.J., Eds.; Springer: New York, NY, USA, 2011; pp. 313–334. [Google Scholar]
- Emami, S.; Azadmard-Damirchi, S.; Peighambardoust, S.H.; Valizadeh, H.; Hesari, J. Liposomes as carrier vehicles for functional compounds in food sector. J. Exp. Nanosci. 2016, 11, 737–759. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to Supercritical Fluids. Processes 2020, 8, 1022. [Google Scholar] [CrossRef]
- Cooper, L.F.; Ravindran, S.; Huang, C.-C.; Kang, M. A Role for Exosomes in Craniofacial Tissue Engineering and Regeneration. Front. Physiol. 2020, 10, 1569. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367. [Google Scholar] [CrossRef]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular Vesicles—Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef] [Green Version]
- Rayamajhi, S.; Aryal, S. Surface functionalization strategies of extracellular vesicles. J. Mater. Chem. B 2020, 8, 4552–4569. [Google Scholar] [CrossRef]
- Lane, R.; Korbie, D.; Hill, M.M.; Trau, M. Extracellular vesicles as circulating cancer biomarkers: Opportunities and challenges. Clin. Transl. Med. 2018, 7, 14. [Google Scholar] [CrossRef]
- Kurian, N.K.; Modi, D. Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy. J. Assist. Reprod. Genet. 2019, 36, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 1–18. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef]
- Hsu, C.; Morohashi, Y.; Yoshimura, S.-I.; Manrique-Hoyos, N.; Jung, S.; Lauterbach, M.A.; Bakhti, M.; Grønborg, M.; Möbius, W.; Rhee, J.; et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A–C. J. Cell Biol. 2010, 189, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.J.; Sims, B.; Matthews, Q.L. Biological Function of Exosomes as Diagnostic Markers and Therapeutic Delivery Vehicles in Carcinogenesis and Infectious Diseases. In Nanomedicines; IntechOpen: London, UK, 2018. [Google Scholar]
- Vietri, M.; Radulovic, M.; Stenmark, H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020, 21, 25–42. [Google Scholar] [CrossRef]
- Patil, A.A.; Rhee, W.J. Exosomes: Biogenesis, Composition, Functions, and Their Role in Pre-metastatic Niche Formation. Biotechnol. Bioprocess. Eng. 2019, 24, 689–701. [Google Scholar] [CrossRef]
- Mathivanan, S.; Fahner, C.J.; Reid, G.; Simpson, R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2011, 40, D1241–D1244. [Google Scholar] [CrossRef] [Green Version]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Akuma, P.; Okagu, O.D.; Udenigwe, C. Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- ElKhoury, K.; Koçak, P.; Kang, A.; Arab-Tehrany, E.; Ward, J.E.; Shin, S.R. Engineering Smart Targeting Nanovesicles and Their Combination with Hydrogels for Controlled Drug Delivery. Pharmaceutics 2020, 12, 849. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Lu, M.; Zhao, X.; Xing, H.; Xun, Z.; Zhu, S.; Lang, L.; Yang, T.; Cai, C.; Wang, D.; Ding, P. Comparison of exosome-mimicking liposomes with conventional liposomes for intracellular delivery of siRNA. Int. J. Pharm. 2018, 550, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, J.; Gu, W.; Huang, Y.; Tong, Z.; Huang, L.; Tan, J. Exosome-Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs. Adv. Sci. 2018, 5, 1700611. [Google Scholar] [CrossRef] [PubMed]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quirk, R.A.; France, R.M.; Shakesheff, K.M.; Howdle, S.M. Supercritical fluid technologies and tissue engineering scaffolds. Curr. Opin. Solid State Mater. Sci. 2004, 8, 313–321. [Google Scholar] [CrossRef]
- Celikkin, N.; Rinoldi, C.; Costantini, M.; Trombetta, M.; Rainer, A.; Święszkowski, W. Naturally derived proteins and glycosaminoglycan scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2017, 78, 1277–1299. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, F.A.; Armentano, I.; Cacciotti, I.; Tiribuzi, R.; Quattrocelli, M.; Del Gaudio, C.; Fortunati, E.; Saino, E.; Caraffa, A.; Cerulli, G.G.; et al. Tuning multi/pluri-potent stem cell fate by electrospun poly(l-lactic acid)-calcium-deficient hydroxyapatite nanocomposite mats. Biomacromolecules 2012, 13, 1350–1360. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Shimojo, A.A.M.; Rodrigues, I.C.P.; Perez, A.G.M.; Souto, E.M.B.; Gabriel, L.P.; Webster, T. Scaffolds for Tissue Engineering: A State-of-the-Art Review Concerning Types, Properties, Materials, Processing, and Characterization. In Racing for the Surface: Antimicrobial and Interface Tissue Engineering; Li, B., Moriarty, T., Webster, T., Xing, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 647–676. [Google Scholar]
- Baranski, J.; Chaturvedi, R.R.; Stevens, K.R.; Eyckmans, J.; Carvalho, B.; Solorzano, R.D.; Yang, M.T.; Miller, J.; Bhatia, S.N.; Chen, C. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc. Natl. Acad. Sci. USA 2013, 110, 7586–7591. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, K.; Akagi, T.; Asaoka, T.; Eguchi, H.; Fukuda, Y.; Iwagami, Y.; Yamada, D.; Noda, T.; Wada, H.; Gotoh, K.; et al. Construction of three-dimensional vascularized functional human liver tissue using a layer-by-layer cell coating technique. Biomaterials 2017, 133, 263–274. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, G.; Palmero, P.; Cacciotti, I.; Pecci, R.; Campagnolo, L.; Bedini, R.; Siracusa, G.; Bianco, A.; Camaioni, A.; Montanaro, L. Design, production and biocompatibility of nanostructured porous HAp and Si-HAp ceramics as three-dimensional scaffolds for stem cell culture and differentiation. Ceramics-Silikaty 2010, 54, 90–96. [Google Scholar]
- Cacciotti, I.; Ciocci, M.; Di Giovanni, E.; Nanni, F.; Melino, S. Hydrogen Sulfide-Releasing Fibrous Membranes: Potential Patches for Stimulating Human Stem Cells Proliferation and Viability under Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 2368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Bioengineered liposome–scaffold composites as therapeutic delivery systems. Ther. Deliv. 2017, 8, 425–445. [Google Scholar] [CrossRef]
- Ahl, P.L.; Bhatia, S.K.; Meers, P.; Roberts, P.; Stevens, R.; Dause, R.; Perkins, W.R.; Janoff, A.S. Enhancement of the in vivo circulation lifetime of l-α-distearoylphosphatidylcholine liposomes: Importance of liposomal aggregation versus complement opsonization. Biochim. et Biophys. Acta BBA Biomembr. 1997, 1329, 370–382. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Mostafa, N.Z.; Incani, V.; Kucharski, C.; Uludağ, H. Bisphosphonate-decorated lipid nanoparticles designed as drug carriers for bone diseases. J. Biomed. Mater. Res. Part. A 2011, 100, 684–693. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, A.; Pires, R.; Faria, S.; Fonseca, N.A.C.; Moreira, J.N.; Reis, R.L.; Neves, N.M. Immobilization of bioactive factor-loaded liposomes on the surface of electrospun nanofibers targeting tissue engineering. Biomater. Sci. 2014, 2, 1195–1209. [Google Scholar] [CrossRef]
- Kulkarni, M.; Breen, A.; Greiser, U.; O’Brien, T.; Pandit, A. Fibrin−Lipoplex System for Controlled Topical Delivery of Multiple Genes. Biomacromolecules 2009, 10, 1650–1654. [Google Scholar] [CrossRef]
- Bengali, Z.; Pannier, A.K.; Segura, T.; Anderson, B.C.; Jang, J.-H.; Mustoe, T.A.; Shea, L.D. Gene delivery through cell culture substrate adsorbed DNA complexes. Biotechnol. Bioeng. 2005, 90, 290–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curley, N.; Levy, D.; Do, M.A.; Brown, A.; Stickney, Z.; Marriott, G.; Lu, B. Sequential deletion of CD63 identifies topologically distinct scaffolds for surface engineering of exosomes in living human cells. Nanoscale 2020, 12, 12014–12026. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, K.; Otaki, N. Bone cell interactions through Eph/ephrin: Bone modeling, remodeling and associated diseases. Cell Adh. Migr. 2012, 6, 148–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.-C.; Yu, T.-T.; Li, J.; Qiao, Y.-Q.; Wang, L.-C.; Zhang, T.; Li, Q.; Zhou, Y.-H.; Liu, D.-W. The Delivery of Extracellular Vesicles Loaded in Biomaterial Scaffolds for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 1015. [Google Scholar] [CrossRef]
- Wong, R.; Tideman, H.; Kin, L.; Merkx, M.A. Biomechanics of mandibular reconstruction: A review. Int. J. Oral Maxillofac. Surg. 2010, 39, 313–319. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Gardin, C.; Zamparini, F.; Ferroni, L.; Degli Esposti, M.; Parchi, G.; Ercan, B.; Manzoli, L.; Fava, F.; Fabbri, P.; et al. Mineral-Doped Poly(L-lactide) Acid Scaffolds Enriched with Exosomes Improve Osteogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells. Nanomaterials 2020, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.K.; Lee, S.; Kim, M.; Jeong, Y.; Lee, S. Exosome-coated silk fibroin 3D-scaffold for inducing osteogenic differentiation of bone marrow derived mesenchymal stem cells. Chem. Eng. J. 2021, 406, 127080. [Google Scholar] [CrossRef]
- Leucht, P.; Lee, S.; Yim, N. Wnt signaling and bone regeneration: Can’t have one without the other. Biomaterials 2019, 196, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Boerckel, J.D.; Kolambkar, Y.M.; Stevens, H.Y.; Lin, A.S.; Dupont, K.M.; Guldberg, R.E. Effects of in vivo mechanical loading on large bone defect regeneration. J. Orthop. Res. 2012, 30, 1067–1075. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Alibolandi, M.; Abnous, K.; Salmasi, Z.; Jaafari, M.R.; Ramezani, M. Fabrication of hybrid scaffold based on hydroxyapatite-biodegradable nanofibers incorporated with liposomal formulation of BMP-2 peptide for bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1987–1997. [Google Scholar] [CrossRef]
- Cui, Z.-K.; Kim, S.; Baljon, J.J.; Doroudgar, M.; LaFleur, M.; Wu, B.M.; Aghaloo, T.; Lee, M. Design and Characterization of a Therapeutic Non-phospholipid Liposomal Nanocarrier with Osteoinductive Characteristics to Promote Bone Formation. ACS Nano 2017, 11, 8055–8063. [Google Scholar] [CrossRef]
- Zarei, F.; Soleimaninejad, M. Role of growth factors and biomaterials in wound healing. Artif. Cells Nanomed. Biotechnol. 2018, 46, 906–911. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.G.; Choi, J.; Kim, K. Mesenchymal Stem Cell-Derived Exosomes for Effective Cartilage Tissue Repair and Treatment of Osteoarthritis. Biotechnol. J. 2020, 15, e2000082. [Google Scholar] [CrossRef]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Gomoll, A.H.; Canseco, J.A.; Far, J.; Lind, M.; Hui, J. Cartilage repair in the degenerative ageing knee. Acta Orthop. 2016, 87, 26–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musumeci, G.; Carnazza, M.L.; Loreto, C.; Leonardi, R.; Loreto, C. β-Defensin-4 (HBD-4) is expressed in chondrocytes derived from normal and osteoarthritic cartilage encapsulated in PEGDA scaffold. Acta Histochem. 2012, 114, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Tian, G.; Yang, Z.; Gao, X.; Wang, F.; Li, J.; Tian, Z.; Huang, B.; Wei, F.; Sang, X.; et al. Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact. Mater. 2021, 6, 2711–2728. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Li, Y.; Niu, X.; Zhao, B.; Wang, Y.; Bao, C.; Xie, Z.; Lin, Q.; Zhu, L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 2017, 9, 4430–4438. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Wang, L.; Yuan, G.; Dai, K.; Pei, J.; Hao, Y. Dual modulation of bone formation and resorption with zoledronic acid-loaded biodegradable magnesium alloy implants improves osteoporotic fracture healing: An in vitro and in vivo study. Acta Biomater. 2018, 65, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Mora-Raimundo, P.; Lozano, D.; Manzano, M.; Vallet-Regí, M. Nanoparticles to Knockdown Osteoporosis-Related Gene and Promote Osteogenic Marker Expression for Osteoporosis Treatment. ACS Nano 2019, 13, 5451–5464. [Google Scholar] [CrossRef] [Green Version]
- Arcos, D.; Boccaccini, A.; Bohner, M.; Diez-Perez, A.; Epple, M.; Gomez-Barrena, E.; Herrera, A.; Planell, J.; Rodríguez-Mañas, L.; Vallet-Regí, M. The relevance of biomaterials to the prevention and treatment of osteoporosis. Acta Biomater. 2014, 10, 1793–1805. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Li, Z.; Zhang, Y.; Lan, F.; He, J.; Wu, Y. The essential role of osteoclast-derived exosomes in magnetic nanoparticle-infiltrated hydroxyapatite scaffold modulated osteoblast proliferation in an osteoporosis model. Nanoscale 2020, 12, 8720–8726. [Google Scholar] [CrossRef]
- Huyan, T.; Du, Y.; Dong, D.; Li, Q.; Zhang, R.; Yang, J.; Yang, Z.; Li, J.; Shang, P. Osteoclast-derived exosomes inhibit osteogenic differentiation through Wnt/β-catenin signaling pathway in simulated microgravity model. Acta Astronaut. 2019, 154, 140–152. [Google Scholar] [CrossRef]
- Miura, M.; Miura, Y.; Sonoyama, W.; Yamaza, T.; Gronthos, S.; Shi, S. Bone marrow-derived mesenchymal stem cells for regenerative medicine in craniofacial region. Oral Dis. 2006, 12, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, V.; Zivkovic, P.; Petrovic, D.; Stefanovic, V. Craniofacial bone tissue engineering. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e1–e9. [Google Scholar] [CrossRef]
- Wei, G.; Jin, Q.; Giannobile, W.; Ma, P.X. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials 2007, 28, 2087–2096. [Google Scholar] [CrossRef] [Green Version]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.B.; Zhang, Z.; Xiu, K.; Gong, T.; Eberle, M.; Wang, Z.; Ma, P.X. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 2020, 118, 215–232. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods; IntechOpen: London, UK, 2017. [Google Scholar]
- Gong, X.; Yang, Y.; Huang, L.; Zhang, Q.; Wan, R.Z.; Zhang, P.; Zhang, B. Antioxidation, anti-inflammation and anti-apoptosis by paeonol in LPS/d-GalN-induced acute liver failure in mice. Int. Immunopharmacol. 2017, 46, 124–132. [Google Scholar] [CrossRef]
- Xia, H.; Cheng, Z.; Xu, Y. Paeonol liposome-hydrogel: Preparation, Penetration through the mouse skin and down-regulation of the expression of tyrosinase. Latin Am. J. Pharm. 2014, 33, 1267–1272. [Google Scholar]
- Freund, P.; Thompson, A.; Curt, A.; Hupp, M.; Weiskopf, N.; Grabher, P.; Altmann, D.; Friston, K.; Ashburner, J.; Ziegler, G. Author response: Progressive neurodegeneration following spinal cord injury: Implications for clinical trials. Neurology 2018, 91, 985. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Dai, J. Bridging the gap with functional collagen scaffolds: Tuning endogenous neural stem cells for severe spinal cord injury repair. Biomater. Sci. 2017, 6, 265–271. [Google Scholar] [CrossRef]
- Li, X.; Fan, C.; Xiao, Z.; Zhao, Y.; Zhang, H.; Sun, J.; Zhuang, Y.; Wu, X.; Shi, J.; Chen, Y.; et al. A collagen microchannel scaffold carrying paclitaxel-liposomes induces neuronal differentiation of neural stem cells through Wnt/β-catenin signaling for spinal cord injury repair. Biomaterials 2018, 183, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Jin, W.; Zhu, T.; Wang, J.; Yuan, B.; Jiang, J.; Liang, W.; Ma, Z. Curcumin modulates TLR4/NF-κB inflammatory signaling pathway following traumatic spinal cord injury in rats. J. Spinal Cord Med. 2015, 38, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Novikova, L.N.; Kolar, M.K.; Kingham, P.; Ullrich, A.; Oberhoffner, S.; Renardy, M.; Doser, M.; Müller, E.; Wiberg, M.; Novikov, L.N. Trimethylene carbonate-caprolactone conduit with poly-p-dioxanone microfilaments to promote regeneration after spinal cord injury. Acta Biomater. 2018, 66, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Altinova, H.; Möllers, S.; Führmann, T.; Deumens, R.; Bozkurt, A.; Heschel, I.; Damink, L.H.O.; Schügner, F.; Weis, J.; Brook, G.A. Functional improvement following implantation of a microstructured, type-I collagen scaffold into experimental injuries of the adult rat spinal cord. Brain Res. 2014, 1585, 37–50. [Google Scholar] [CrossRef]
- Caicco, M.J.; Zahir, T.; Mothe, A.J.; Ballios, B.G.; Kihm, A.J.; Tator, C.H.; Shoichet, M.S. Characterization of hyaluronan-methylcellulose hydrogels for cell delivery to the injured spinal cord. J. Biomed. Mater. Res. Part A 2012, 101, 1472–1477. [Google Scholar] [CrossRef]
- Wang, X.; Botchway, B.O.A.; Zhang, Y.; Yuan, J.; Liu, X. Combinational Treatment of Bioscaffolds and Extracellular Vesicles in Spinal Cord Injury. Front. Mol. Neurosci. 2019, 12, 81. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.-T.; Tong, D.-M.; Wang, S.-D.; Ye, S.; Xu, B.-W.; Yang, C.-X. Acute spontaneous intracerebral hemorrhage and traumatic brain injury are the most common causes of critical illness in the ICU and have high early mortality. BMC Neurol. 2018, 18, 127. [Google Scholar] [CrossRef]
- Yuan, J.; Botchway, B.O.A.; Zhang, Y.; Wang, X.; Liu, X. Combined bioscaffold with stem cells and exosomes can improve traumatic brain injury. Stem Cell Rev. Rep. 2019, 16, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, B.E.; Maas, A.I.R.; Narayan, R.K.; Van Der Hoop, R.G.; MacAllister, T.; Ward, J.D.; Nelson, N.R.; Stocchetti, N. A Clinical Trial of Progesterone for Severe Traumatic Brain Injury. N. Engl. J. Med. 2014, 371, 2467–2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escrevente, C.; Keller, S.; Altevogt, P.; Costa, J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 2011, 11, 108. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-K.; Nishida, H.; An, S.Y.; Shetty, A.; Bartosh, T.J.; Prockop, D.J. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci. USA 2016, 113, 170–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chopp, M.; Zhang, Z.G.; Katakowski, M.; Xin, H.; Qu, C.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 2017, 111, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Soller, E.C.; Tzeranis, D.; Miu, K.; So, P.T.; Yannas, I.V. Common features of optimal collagen scaffolds that disrupt wound contraction and enhance regeneration both in peripheral nerves and in skin. Biomaterials 2012, 33, 4783–4791. [Google Scholar] [CrossRef] [PubMed]
- Melling, G.E.; Colombo, J.S.; Avery, S.J.; Ayre, W.N.; Evans, S.L.; Waddington, R.J.; Sloan, A. Liposomal Delivery of Demineralized Dentin Matrix for Dental Tissue Regeneration. Tissue Eng. Part A 2018, 24, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Taylor, E.; Gomel, V. The uterus and fertility. Fertil. Steril. 2008, 89, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wong, Y.M.; Cheong, Y.; Xia, E.; Li, T.C. Asherman syndrome—One century later. Fertil. Steril. 2008, 89, 759–779. [Google Scholar] [CrossRef]
- Wallach, E.E.; Schenker, J.G.; Margalioth, E.J. Intrauterine adhesions: An updated appraisal. Fertil. Steril. 1982, 37, 593–610. [Google Scholar] [CrossRef]
- Xin, L.; Lin, X.; Zhou, F.; Li, C.; Wang, X.; Yu, H.; Pan, Y.; Fei, H.; Ma, L.; Zhang, S. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomater. 2020, 113, 252–266. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, H.; Zhu, H.; Zhu, X.; Tang, X.; Yan, G.; Wang, J.; Bai, D.; Wang, J.; Wang, L.; et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: A phase I clinical trial. Stem Cell Res. Ther. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saribas, G.S.; Ozogul, C.; Tiryaki, M.; Pinarli, F.A.; Kilic, S.H. Effects of uterus derived mesenchymal stem cells and their exosomes on asherman’s syndrome. Acta Histochem. 2020, 122, 151465. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Cheung, K.J.; Gabrielson, E.; Werb, Z.; Ewald, A.J. Collective Invasion in Breast Cancer Requires a Conserved Basal Epithelial Program. Cell 2013, 155, 1639–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, S.-Y.; Zhang, A.-Q.; Cheng, S.-X.; Rong, L.; Zhang, X.-Z. Drug self-delivery systems for cancer therapy. Biomaterials 2017, 112, 234–247. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Alimbetov, D.; Askarova, S.; Umbayev, B.; Davis, T.; Kipling, D. Pharmacological Targeting of Cell Cycle, Apoptotic and Cell Adhesion Signaling Pathways Implicated in Chemoresistance of Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Sugarman, R.; Patel, R.; Sharma, S.; Plenker, D.; Tuveson, D.; Saif, M.W. Pharmacokinetics and pharmacodynamics of new drugs for pancreatic cancer. Expert Opin. Drug Metab. Toxicol. 2019, 15, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Mickova, A.; Buzgo, M.; Benada, O.; Rampichova, M.; Fišar, Z.; Filova, E.; Tesarova, M.; Lukáš, D.; Amler, E. Core/Shell Nanofibers with Embedded Liposomes as a Drug Delivery System. Biomacromolecules 2012, 13, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Hou, J.; Yang, G.; Zhou, S. A Time-Programmed Release of Dual Drugs from an Implantable Trilayer Structured Fiber Device for Synergistic Treatment of Breast Cancer. Small 2020, 16, e1902262. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Du, B.; Chen, Y.; Song, N.; Li, Z.; Li, J.; Luo, F.; Tan, H. Dual-encapsulated biodegradable 3D scaffold from liposome and waterborne polyurethane for local drug control release in breast cancer therapy. J. Biomater. Sci. Polym. Ed. 2020, 31, 2220–2237. [Google Scholar] [CrossRef]
- Mao, Y.; Li, X.; Chen, G.; Wang, S. Thermosensitive Hydrogel System with Paclitaxel Liposomes Used in Localized Drug Delivery System for In Situ Treatment of Tumor: Better Antitumor Efficacy and Lower Toxicity. J. Pharm. Sci. 2016, 105, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Chen, H.; Li, S.; Guo, X. In vitro and in vivo investigation of a novel two-phase delivery system of 2-methoxyestradiol liposomes hydrogel. J. Liposome Res. 2014, 24, 10–16. [Google Scholar] [CrossRef]
- Salsali, A.; Nathan, M. A Review of Types 1 and 2 Diabetes Mellitus and Their Treatment with Insulin. Am. J. Ther. 2006, 13, 349–361. [Google Scholar] [CrossRef]
- Chen, X.; Wong, B.C.K.; Chen, H.; Zhang, S.; Bian, Z.; Zhang, G.; Lin, C.; Riaz, M.K.; Tyagi, D.; Lu, A.; et al. Long-lasting Insulin Treatment Via a Single Subcutaneous Administration of Liposomes in Thermoreversible Pluronic® F127 Based Hydrogel. Curr. Pharm. Des. 2018, 23, 6079–6085. [Google Scholar] [CrossRef]
- Haque, M.R.; Lee, D.Y.; Ahn, C.-H.; Jeong, J.-H.; Byun, Y. Local Co-Delivery of Pancreatic Islets and Liposomal Clodronate Using Injectable Hydrogel to Prevent Acute Immune Reactions in a Type 1 Diabetes. Pharm. Res. 2014, 31, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Efron, P.A.; Moore, F.A.; Brakenridge, S.C. Persistent Inflammation, Immunosuppression and Catabolism after Severe Injury or Infection. In Annual Update in Intensive Care and Emergency Medicine 2018; Vincent, J.-L., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 25–35. [Google Scholar]
- Negi, P.; Aggarwal, M.; Sharma, G.; Rathore, C.; Sharma, G.; Singh, B.; Katare, O. Niosome-based hydrogel of resveratrol for topical applications: An effective therapy for pain related disorder(s). Biomed. Pharmacother. 2017, 88, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Brew, B.J.; Garber, J.Y. Neurologic sequelae of primary HIV infection. Hum. Hypothal. Middle Posterior Reg. 2018, 152, 65–74. [Google Scholar] [CrossRef]
- Ramanathan, R.; Jiang, Y.; Read, B.; Golan-Paz, S.; Woodrow, K. Biophysical characterization of small molecule antiviral-loaded nanolipogels for HIV-1 chemoprophylaxis and topical mucosal application. Acta Biomater. 2016, 36, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Esposito, F.; Ambrosio, F.A.; Maleddu, R.; Costa, G.; Rocca, R.; Maccioni, E.; Catalano, R.; Romeo, I.; Eleftheriou, P.; Karia, D.C.; et al. Chromenone derivatives as a versatile scaffold with dual mode of inhibition of HIV-1 reverse transcriptase-associated Ribonuclease H function and integrase activity. Eur. J. Med. Chem. 2019, 182, 111617. [Google Scholar] [CrossRef]
- Lang, S.; Kansy, B. Cervical lymph node diseases in children. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2014, 13, 08. [Google Scholar] [CrossRef]
- Hurler, J.; Sørensen, K.K.; Fallarero, A.; Vuorela, P.; Škalko-Basnet, N. Liposomes-in-Hydrogel Delivery System with Mupirocin: In Vitro Antibiofilm Studies and In Vivo Evaluation in Mice Burn Model. BioMed Res. Int. 2013, 2013, 498485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.-T.; Xu, Y.-Q.; Shi, J.; Li, J.; Ding, J. Liposome combined porous β-TCP scaffold: Preparation, characterization, and anti-biofilm activity. Drug Deliv. 2010, 17, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Homann, H.-H.; Rosbach, O.; Moll, W.; Vogt, P.M.; Germann, G.; Hopp, M.; Langer-Brauburger, B.; Reimer, K.; Steinau, H.-U. A Liposome Hydrogel With Polyvinyl-Pyrrolidone Iodine in the Local Treatment of Partial-Thickness Burn Wounds. Ann. Plast. Surg. 2007, 59, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-L.; Chen, P.-P.; Zhuge, D.-L.; Zhu, Q.-Y.; Jin, B.-H.; Shen, B.-X.; Xiao, J.; Zhao, Y.-Z. Liposomes with Silk Fibroin Hydrogel Core to Stabilize bFGF and Promote the Wound Healing of Mice with Deep Second-Degree Scald. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Berg, O.A.; Skar, M.; Conradi, A.H.; Johnsen, P.J.; Škalko-Basnet, N. Improved Burns Therapy: Liposomes-in-Hydrogel Delivery System for Mupirocin. J. Pharm. Sci. 2012, 101, 3906–3915. [Google Scholar] [CrossRef] [PubMed]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and Physiology of Wound Healing. Clin. Plast. Surg. 2012, 39, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Baglio, S.R.; Pegtel, D.M.; Baldini, N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 2012, 3, 359. [Google Scholar] [CrossRef] [Green Version]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Cooper, D.R.; Wang, C.; Patel, R.; Trujillo, A.; Patel, N.A.; Prather, J.; Gould, L.J.; Wu, M.H. Human Adipose-Derived Stem Cell Conditioned Media and Exosomes Containing MALAT1 Promote Human Dermal Fibroblast Migration and Ischemic Wound Healing. Adv. Wound Care 2018, 7, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Taghdiri Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. J. Biomed. Mater. Res. Part A 2020, 108, 545–556. [Google Scholar] [CrossRef]
- Liu, C.; Su, C. Design strategies and application progress of therapeutic exosomes. Theranostics 2019, 9, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.-A.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Griffith, L.G.; Naughton, G. Tissue engineering—Current challenges and expanding opportunities. Science 2002, 295, 1009–1014. [Google Scholar]
- Chapekar, M.S. Tissue engineering: Challenges and opportunities. J. Biomed. Mater. Res. 2000, 53, 617–620. [Google Scholar] [CrossRef]
- Langer, R. Tissue engineering: A new field and its challenges. Pharm. Res. 1997, 14, 840–841. [Google Scholar] [CrossRef] [PubMed]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Paul, A.; Vrana, N.E.; Zhao, X.; Memic, A.; Hwang, Y.-S.; Dokmeci, M.R.; Khademhosseini, A. Microfluidic techniques for development of 3D vascularized tissue. Biomaterials 2014, 35, 7308–7325. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopra, N.; Arya, B.D.; Jain, N.; Yadav, P.; Wajid, S.; Singh, S.P.; Choudhury, S. Biophysical Characterization and Drug Delivery Potential of Exosomes from Human Wharton’s Jelly-Derived Mesenchymal Stem Cells. ACS Omega 2019, 4, 13143–13152. [Google Scholar] [CrossRef] [Green Version]
- Lai, R.C.; Tan, S.S.; Teh, B.J.; Sze, S.K.; Arslan, F.; De Kleijn, D.P.; Choo, A.; Lim, S.K. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int. J. Proteom. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zha, Y.; Li, Y.; Lin, T.; Chen, J.; Zhang, S.; Wang, J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics 2021, 11, 397–409. [Google Scholar] [CrossRef]
- Ma, Y.-H.; Yang, J.; Li, B.; Jiang, Y.-W.; Lu, X.; Chen, Z. Biodegradable and injectable polymer–liposome hydrogel: A promising cell carrier. Polym. Chem. 2016, 7, 2037–2044. [Google Scholar] [CrossRef]






| Lamellarity | Abbreviation | Number of Lipid Bilayers | Diameter Size Rang Structures | |
|---|---|---|---|---|
| (1) Unilamellar Vesicles | ULV | one lipid bilayer | All size range |  |
| Dimensions | ||||
| Name | Abbreviation | Size Range (µm) | Characteristics | |
| Single Unilamellar Vesicles | SUV | 0.02–0.20 | useful for entrapping lipophilic active materials | |
| Medium Unilamellar Vesicles | MUV | 0.20–0.50 | slightly more efficient than SUV | |
| Large Unilamellar Vesicles | LUV | 0.50–10 | capable of capturing a significant amount of hydrophilic material | |
| Giant Unilamellar Vesicles | GUV | 100–200 | look like cell membranes, ideal templates for microscale bioreactors | |
| (2) Oligolamellar Vesicles | OLV | few concentric lipid bilayers | 100–500 nm |  |
| (3) Multilamellar Vesicles | MLV | many concentric lipid bilayers | >500 nm |  |
| (4) Multivesicular Vesicles | MVV | non-concentric vesicles within a single lipid bilayer | >1000 nm |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafiei, M.; Ansari, M.N.M.; Razak, S.I.A.; Khan, M.U.A. A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering. Polymers 2021, 13, 2529. https://doi.org/10.3390/polym13152529
Shafiei M, Ansari MNM, Razak SIA, Khan MUA. A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering. Polymers. 2021; 13(15):2529. https://doi.org/10.3390/polym13152529
Chicago/Turabian StyleShafiei, Mojtaba, Mohamed Nainar Mohamed Ansari, Saiful Izwan Abd Razak, and Muhammad Umar Aslam Khan. 2021. "A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering" Polymers 13, no. 15: 2529. https://doi.org/10.3390/polym13152529