Water-Borne ZnO/Acrylic Nanocoating: Fabrication, Characterization, and Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Fabrication
2.3. Coating Characterization
2.4. Weathering Aging Test
2.5. Self-Cleaning Test
3. Results and Discussion
3.1. Effect of ZnO Particles on the Mechanical Property of a Water-Borne Acrylic Coating
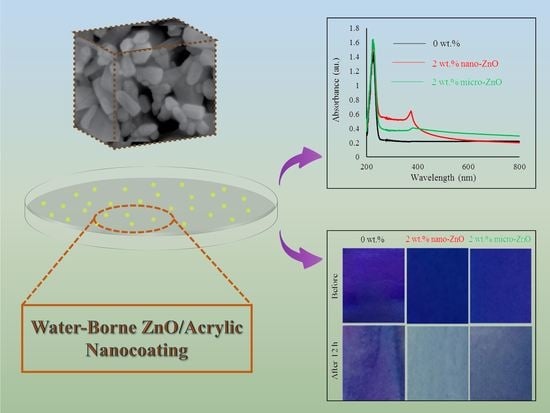
3.2. Effect of ZnO Particles on the Accelerated Weathering Aging of Water-Borne Acrylic Coating
3.3. Self-Cleaning Performance of Water-Borne Acrylic Coating Containing ZnO Particles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izmitli, A.; Ngunjiri, J.; Lan, T.; Pacholski, M.L.; Smith, R.; Langille, M.; Roggow, T.; Henderson, K.; Kalantar, T.; Manna, J. Impact of silicone additives on slip/mar performance and surface characteristics of waterborne acrylic coatings. Prog. Org. Coat. 2019, 131, 145–151. [Google Scholar] [CrossRef]
- Saha, S.; Kocaefe, D.; Krause, C.; Larouche, T. Effect of titania and zinc oxide particles on acrylic polyurethane coating performance. Prog. Org. Coat. 2011, 70, 170–177. [Google Scholar] [CrossRef]
- Cogulet, A.; Blanchet, P.; Landry, V. Evaluation of the impacts of four weathering methods on two acrylic paints: Showcasing distinctions and particularities. Coatings 2019, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Bellotti, N.; Romagnoli, R.; Quintero, C.; Domínguez-Wong, C.; Ruiz, F.; Deyá, C. Nanoparticles as antifungal additives for indoor waterborne paints. Prog. Org. Coat. 2015, 86, 33–40. [Google Scholar] [CrossRef]
- Pinho, L.; Rojas, M.; Mosquera, M.J. Ag–SiO2–TiO2 nanocomposite coatings with enhanced photoactivity for self-cleaning application on building materials. Appl. Catal. B Environ. 2015, 178, 144–154. [Google Scholar] [CrossRef]
- Yasin, G.; Arif, M.; Mehtab, T.; Lu, X.; Yu, D.; Muhammad, N.; Nazir, M.T.; Song, H. Understanding and suppression strategies toward stable Li metal anode for safe lithium batteries. Energy Storage Mater. 2020, 25, 644–678. [Google Scholar] [CrossRef]
- Tabish, M.; Yasin, G.; Anjum, M.J.; Malik, M.U.; Zhao, J.; Yang, Q.; Manzoor, S.; Murtaza, H.; Khan, W.Q. Reviewing the current status of layered double hydroxide-based smart nanocontainers for corrosion inhibiting applications. J. Mater. Res. Technol. 2021, 10, 390–421. [Google Scholar] [CrossRef]
- Tabish, M.; Malik, M.U.; Khan, M.A.; Yasin, G.; Asif, H.M.; Anjum, M.J.; Khan, W.Q.; Ibraheem, S.; Nguyen, T.A.; Slimani, Y. Construction of NiCo/Graphene Nanocomposite Coating with Bulges-like Morphology for Enhanced Mechanical Properties and Corrosion Resistance Performance. J. Alloys Compd. 2021, 867, 159138. [Google Scholar] [CrossRef]
- Dillon, C.E.; Lagalante, A.F.; Wolbers, R.C. Acrylic emulsion paint films: The effect of solution pH, conductivity, and ionic strength on film swelling and surfactant removal. Stud. Conserv. 2014, 59, 52–62. [Google Scholar] [CrossRef]
- Özgenç, Ö. Comparison of durability of wood coatings containing different waterborne acrylic resins and UV absorbers in natural weathering. Drew. Pr. Nauk. Doniesienia Komun. 2020, 62, 47–61. [Google Scholar]
- Bui, T.M.A.; Nguyen, T.V.; Nguyen, T.M.; Hoang, T.H.; Nguyen, T.T.H.; Lai, T.H.; Tran, T.N.; Hoang, V.H.; Le, T.L.; Dang, T.C. Investigation of crosslinking, mechanical properties and weathering stability of acrylic polyurethane coating reinforced by SiO2 nanoparticles issued from rice husk ash. Mater. Chem. Phys. 2020, 241, 122445. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Nguyen-Tri, P.; Azizi, S.; Dang, T.C.; Hoang, D.M.; Hoang, T.H.; Nguyen, T.L.; Le Bui, T.T.; Dang, V.H.; Nguyen, N.L. The role of organic and inorganic UV-absorbents on photopolymerization and mechanical properties of acrylate-urethane coating. Mater. Today Commun. 2020, 22, 100780. [Google Scholar] [CrossRef]
- Yasin, G.; Arif, M.; Mehtab, T.; Shakeel, M.; Khan, M.A.; Khan, W.Q. Metallic nanocomposite coatings. In Corrosion Protection at the Nanoscale; Elsevier: Amsterdam, The Netherlands, 2020; pp. 245–274. [Google Scholar]
- Nguyen, V.T.; Tabish, M.; Yasin, G.; Bilal, M.; Nguyen, T.H.; Van, C.P.; Nguyen-Tri, P.; Gupta, R.K.; Nguyen, T.A. A facile strategy for the construction of TiO2/Ag nanohybrid-based polyethylene nanocomposite for antimicrobial applications. Nano-Struct. Nano-Obj. 2021, 25, 100671. [Google Scholar] [CrossRef]
- Sbardella, F.; Bracciale, M.P.; Santarelli, M.L.; Asua, J.M. Waterborne modified-silica/acrylates hybrid nanocomposites as surface protective coatings for stone monuments. Prog. Org. Coat. 2020, 149, 105897. [Google Scholar] [CrossRef]
- Bal, A.; Güçlü, G.; İyim, T.B.; Özgümüş, S. Effects of nanoparticles on film properties of waterborne acrylic emulsions. Polym. Plast. Technol. Eng. 2011, 50, 990–995. [Google Scholar] [CrossRef]
- Miklečić, J.; Turkulin, H.; Jirouš-Rajković, V. Weathering performance of surface of thermally modified wood finished with nanoparticles-modified waterborne polyacrylate coatings. Appl. Surf. Sci. 2017, 408, 103–109. [Google Scholar] [CrossRef]
- Miklečić, J.; Blagojević, S.L.; Petrič, M.; Jirouš-Rajković, V. Influence of TiO2 and ZnO nanoparticles on properties of waterborne polyacrylate coating exposed to outdoor conditions. Prog. Org. Coat. 2015, 89, 67–74. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Dao, P.H.; Duong, K.L.; Duong, Q.H.; Vu, Q.T.; Nguyen, A.H.; Le, T.L. Effect of R-TiO2 and ZnO nanoparticles on the UV-shielding efficiency of water-borne acrylic coating. Prog. Org. Coat. 2017, 110, 114–121. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Dao, P.H.; Nguyen, T.A.; Dang, V.H.; Ha, M.N.; Nguyen, T.T.T.; Vu, Q.T.; Nguyen, N.L.; Dang, T.C.; Nguyen-Tri, P. Photocatalytic degradation and heat reflectance recovery of waterborne acrylic polymer/ZnO nanocomposite coating. J. Appl. Polym. Sci. 2020, 137, 49116. [Google Scholar] [CrossRef]
- Javadi, E.; Ghaffari, M.; Bahlakeh, G.; Taheri, P. Photocatalytic, corrosion protection and adhesion properties of acrylic nanocomposite coating containing silane treated nano zinc oxide: A combined experimental and simulation study. Prog. Org. Coat. 2019, 135, 496–509. [Google Scholar] [CrossRef]
- Suwanboon, S.; Amornpitoksuk, P.; Randorn, C. Effect of tartaric acid as a structure-directing agent on different ZnO morphologies and their physical and photocatalytic properties. Ceram. Int. 2019, 45, 2111–2116. [Google Scholar] [CrossRef]
- Chen, X.D.; Wang, Z.; Liao, Z.F.; Mai, Y.L.; Zhang, M.Q. Roles of anatase and rutile TiO2 nanoparticles in photooxidation of polyurethane. Polym. Test. 2007, 26, 202–208. [Google Scholar] [CrossRef]
- Hien, L.X.; Vuong, N.T. Study of the influence of the chemical nature on the humid heat, ultraviolet radiation durability of some coatings based on acrylic resins. Vietnam J. Sci. Technol. 2009, 47, 69–75. [Google Scholar]
- Mac Van Phuc, N.T.V.; Hung, D.P.; Hiep, N.A.; Van Thanh, T. Accelerated aging of solar heat reflective coating on acrylic emulsion. Vietnam J. Chem. 2016, 54, 286–292. [Google Scholar]
- Larché, J.F.; Bussière, P.O.; Gardette, J.L. How to reveal latent degradation of coatings provoked by UV-light. Polym. Degrad. Stab. 2010, 95, 1810–1817. [Google Scholar] [CrossRef]
- Fufa, S.M.; Jelle, B.P.; Hovde, P.J. Weathering performance of spruce coated with water based acrylic paint modified with TiO2 and clay nanoparticles. Prog. Org. Coat. 2013, 76, 1543–1548. [Google Scholar] [CrossRef]
- Özgenç, Ö.; Durmaz, S.; Şahin, S.; Boyaci, I.H. Evaluation of the weathering resistance of waterborne acrylic-and alkyd-based coatings containing HALS, UV absorber, and bark extracts on wood surfaces. J. Coat. Technol. Res. 2020, 17, 461–475. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Zukowska, G.Z.; Korczagin, I.; Malanowski, P. Effect of TiO2 on UV stability of polymeric binder films used in waterborne facade paints. Prog. Org. Coat. 2015, 85, 123–130. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Sabzi, M.; Zohuriaan-Mehr, J.; Atai, M.; Behzadnasab, M. Weathering performance of the polyurethane nanocomposite coatings containing silane treated TiO2 nanoparticles. Appl. Surf. Sci. 2011, 257, 4196–4203. [Google Scholar] [CrossRef]
- Bonnefond, A.; González, E.; Asua, J.M.; Leiza, J.R.; Ieva, E.; Brinati, G.; Carella, S.; Marrani, A.; Veneroni, A.; Kiwi, J. Stable photocatalytic paints prepared from hybrid core-shell fluorinated/acrylic/TiO2 waterborne dispersions. Crystals 2016, 6, 136. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.V.; Tri, P.N.; Nguyen, T.D.; El Aidani, R.; Decker, C. Accelerated degradation of water borne acrylic nanocomposites used in outdoor protective coatings. Polym. Degrad. Stab. 2016, 128, 65–76. [Google Scholar] [CrossRef]
- Le, T.T.; Nguyen, T.V.; Nguyen, T.A.; Nguyen, T.T.H.; Thai, H.; Dinh, D.A.; Nguyen, T.M. Thermal, mechanical and antibacterial properties of water-based acrylic Polymer/SiO2–Ag nanocomposite coating. Mater. Chem. Phys. 2019, 232, 362–366. [Google Scholar] [CrossRef]
- Vuong, N.T.; Linh, N.T. The accelerated weathering aging of a water-borne styrene acrylic coating. Vietnam J. Chem. 2016, 54, 139. [Google Scholar]
- Kardar, P.; Ebrahimi, M.; Bastani, S. Study the effect of nano-alumina particles on physical–mechanical properties of UV cured epoxy acrylate via nano-indentation. Prog. Org. Coat. 2008, 62, 321–325. [Google Scholar] [CrossRef]
- Seentrakoon, B.; Junhasavasdikul, B.; Chavasiri, W. Enhanced UV-protection and antibacterial properties of natural rubber/rutile-TiO2 nanocomposites. Polym. Degrad. Stab. 2013, 98, 566–578. [Google Scholar] [CrossRef]
- Hoa, T.T.; Hoa, L.T.; Khieu, D.Q.; Son, L.C. Study on photooxydation reaction of methylene blue on nano TiO2 catalyst under the effect of sunlight. Hue Univ. J. Sci. 2011, 65, 88–93. [Google Scholar]
- Peter, A.; Mihaly-Cozmuta, A.; Nicula, C.; Mihaly-Cozmuta, L.; Jastrzębska, A.; Olszyna, A.; Baia, L. UV Light-Assisted Degradation of Methyl Orange, Methylene Blue, Phenol, Salicylic Acid, and Rhodamine B: Photolysis Versus Photocatalyis. Water Air. Soil. Pollut. 2016, 228, 41. [Google Scholar] [CrossRef]
- Che Ramli, Z.A.; Asim, N.; Isahak, W.N.R.W.; Emdadi, Z.; Ahmad-Ludin, N.; Yarmo, M.A.; Sopian, K. Photocatalytic Degradation of Methylene Blue under UV Light Irradiation on Prepared Carbonaceous. Sci. World J. 2014, 2014, 415136. [Google Scholar] [CrossRef] [Green Version]
- Oda, A.M.; Salih, A.; Hadi, S.; Jawad, A.; Sadoon, A.; Fahim, Y.; Fadhil, A. Photocatalytic decolorization of methylene blue dye by zinc oxide powder. J. Babylon. Univ. Pure Appl. Sci. 2014, 22, 2508–2515. [Google Scholar]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, T.V.; Nguyen, T.V.; Tabish, M.; Ibrahim, S.; Hoang, T.H.T.; Gupta, R.K.; Dang, T.M.L.; Nguyen, T.A.; Yasin, G. Water-Borne ZnO/Acrylic Nanocoating: Fabrication, Characterization, and Properties. Polymers 2021, 13, 717. https://doi.org/10.3390/polym13050717
Vu TV, Nguyen TV, Tabish M, Ibrahim S, Hoang THT, Gupta RK, Dang TML, Nguyen TA, Yasin G. Water-Borne ZnO/Acrylic Nanocoating: Fabrication, Characterization, and Properties. Polymers. 2021; 13(5):717. https://doi.org/10.3390/polym13050717
Chicago/Turabian StyleVu, Tien Viet, Thien Vuong Nguyen, Mohammad Tabish, Sehrish Ibrahim, Thi Huong Thuy Hoang, Ram K. Gupta, Thi My Linh Dang, Tuan Anh Nguyen, and Ghulam Yasin. 2021. "Water-Borne ZnO/Acrylic Nanocoating: Fabrication, Characterization, and Properties" Polymers 13, no. 5: 717. https://doi.org/10.3390/polym13050717