Free-Radical Photopolymerization of Acrylonitrile Grafted onto Epoxidized Natural Rubber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation and Characterizations
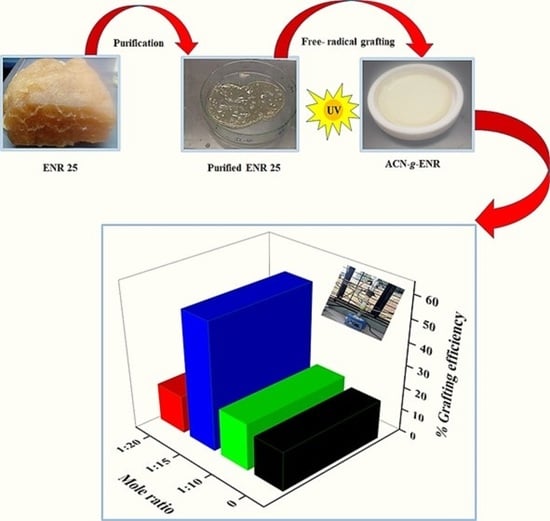
2.2.1. Purification of Reagents
2.2.2. UV Photopolymerization of ACN onto ENR- 25
2.2.3. Purification of ACN-g-ENR Products
2.2.4. Mechanism of Reaction
2.3. Characterizations
2.3.1. Fourier Transform Attenuated Total Reflection (FR-ATR)
2.3.2. Nuclear Magnetic Resonance (NMR)
2.3.3. Gel Permeation Chromatography (GPC)
2.3.4. Grafting and Crosslinking Studies
2.3.5. Dynamic Mechanical Analysis (DMA)
2.3.6. Differential Scanning Calorimetry (DSC)
2.3.7. Thermogravimetry Analysis (TGA) and Derivation of Thermogravimetric (DTG) Analyses
2.3.8. Dielectric Spectroscopy Study (DSS)
3. Results and Discussion
3.1. Chemical interaction
3.2. Molecular Weight Distribution Measurements (MWDM)
3.3. Grafting Efficiency and Crosslinking Density
3.4. Thermal Analyses
3.5. Dielectric Spectroscopy Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Statistics Malaysia, Monthly Rubber Statistics Malaysia, January 2020. Available online: https://www.dosm.gov.my/v1/index.php?r=column/cthemeByCat&cat=73&bul_id=NS85MWFLdDNDb2RWUkpJY09nYWlVQT09&menu_id=Z0VTZGU1UHBUT1VJMFlpaXRRR0xpdz09 (accessed on 13 March 2020).
- Department of Statistics Malaysia, Monthly Rubber Statistics Malaysia, February 2020. Available online: https://www.dosm.gov.my/v1/index.php?r=column/cthemeByCat&cat=73&bul_id=RmtHZ1F0dWpwd2VYSWcyajBFakVqdz09&menu_id=Z0VTZGU1UHBUT1VJMFlpaXRRR0xpdz09 (accessed on 15 April 2020).
- Cornish, K. Alternative natural rubber crops: Why should we care? Technol. Innov. 2017, 18, 244–255. [Google Scholar] [CrossRef]
- Rooshenass, P.; Yahya, R.; Gan, S.N. Comparison of three different degradation methods to produce liquid epoxidized natural rubber. Rubber Chem. Technol. 2016, 89, 177–198. [Google Scholar] [CrossRef] [Green Version]
- Salaeh, S. Processing of Natural Rubber Composites and Blends: Relation between Structure and Properties. Ph.D. Thesis, Université Claude Bernard Lyon 1, Villeurbanne, France, Prince of Songkla University, Pattani, Thailand, 4 July 2014. [Google Scholar]
- Ataollahi, N.; Ahmad, A.; Lee, T.K.; Abdullah, A.; Rahman, M. Preparation and characterization of PVDF-MG49-NH4CF3SO3 based solid polymer electrolyte. e-Polymers 2014, 14, 115–120. [Google Scholar] [CrossRef]
- Harun, F.; Chan, C.H. Electronic Applications of Polymer Electrolytes of Epoxidized Natural Rubber and Its Composites; Springer: Cham, Switzerland, 2016; pp. 37–59. [Google Scholar]
- Srirachya, N.; Kobayashi, T.; Boonkerd, K. An alternative crosslinking of epoxidized natural rubber with maleic anhydride. Key Eng. Mater. 2017, 748, 84–90. [Google Scholar] [CrossRef]
- Stephens, H.L.; Bhowmick, A.K. (Eds.) Handbook of Elastomers; Marcel Dekker, Inc.: Totnes, UK, 2001. [Google Scholar]
- Lee, T.K.; Afiqah, S.; Ahmad, A.; Dahlan, H.M.; Rahman, M.Y.A. Temperature dependence of the conductivity of plasticized poly(vinyl chloride)-low molecular weight liquid 50% epoxidized natural rubber solid polymer electrolyte. J. Solid State Electrochem. 2012, 16, 2251–2260. [Google Scholar] [CrossRef]
- Rahman, M.Y.A.; Ahmad, A.; Lee, T.K.; Farina, Y.; Dahlan, H.M. Effect of ethylene carbonate (EC) plasticizer on poly(vinyl chloride)-liquid 50% epoxidised natural rubber (LENR50) based polymer electrolyte. Mater. Sci. Appl. 2011, 2, 817–825. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.Y.A.; Ahmad, A.; Lee, T.K.; Farina, Y.; Dahlan, H.M. LiClO4 salt concentration effect on the properties of PVC-modified low molecular weight LENR50-based solid polymer electrolyte. J. Appl. Polym. Sci. 2012, 124, 2227–2233. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Allonas, X.; Burget, D. Photopolymerization reactions under visible lights: Principle, mechanisms and examples of applications. Prog. Org. Coatings 2003, 47, 16–36. [Google Scholar] [CrossRef]
- Ates, S.; Aydogan, B.; Torun, L.; Yagci, Y. Synthesis and characterization of triptycene type cross-linker and its use in photoinduced curing applications. Polymer 2010, 51, 825–831. [Google Scholar] [CrossRef]
- Dizman, C.; Ates, S.; Torun, L.; Yagci, Y. Synthesis, characterization and photoinduced curing of polysulfones with (meth)acrylate functionalities. Beilstein J. Org. Chem. 2010, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Mishra, M.; Yagci, Y. Handbook of Vinyl Polymers: Radical Polymerization, Process, and Technology; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Kookarinrat, C.; Paoprasert, P. Versatile one-pot synthesis of grafted-hydrogenated natural rubber. Iran. Polym. J. 2015, 24, 123–133. [Google Scholar] [CrossRef]
- Van Zyl, A.J.; Graef, S.M.; Sanderson, R.D.; Klumperman, B.; Pasch, H. Monitoring the grafting of epoxidized natural rubber by size-exclusion chromatography coupled to FTIR spectroscopy. J. Appl. Polym. Sci. 2003, 88, 2539–2549. [Google Scholar] [CrossRef]
- Moolsin, S.; Robishaw, N.K. Natural rubber modification by vinyl monomers grafting: A review. Rangsit J. Art Sci. 2011, 5, 99–116. [Google Scholar]
- Ratnam, C.T.; Nasir, M.; Baharin, A.; Zaman, K. Effect of blending parameters on electron beam enhancement of PVC/ENR blends. Polym. Plast. Technol. Eng. 2001, 40, 561–575. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, Q.; Liu, J.; Gao, Y.; Sun, Y.; Zhang, Q. Preparation and Characterization of Grafted Natural Rubber/Graphene Oxide Nanocomposites. J. Macromol. Sci. Part B 2019, 58, 645–658. [Google Scholar] [CrossRef]
- Idris, R.; Bujang, N.H. Epoxidised Natural Rubber Based Polymer Electrolyte Systems for Electrochemical Device Applications. Adv. Mater. Res. 2014, 896, 62–65. [Google Scholar] [CrossRef]
- Abd El Salam, H.M.; Sayyh, S.M.; Kamal, E.H.M. Enhancing both the mechanical and chemical properties of paper sheet by graft co-polymerization with acrylonitrile/methyl methacrylate. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 193–202. [Google Scholar]
- Athawale, V.D.; Lele, V. Syntheses and characterisation of graft copolymers of maize starch and methacrylonitrile. Carbohydr. Polym. 2000, 41, 407–416. [Google Scholar] [CrossRef]
- Casinos, I. Mechanisms for the radical graft polymerization of vinyl and/or acrylic monomers on cellulose. Polymer 1994, 35, 606–615. [Google Scholar]
- Bhattacharya, A.; Rawlins, J.W.; Ray, P. Polymer Grafting and Crosslinking; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Pérez, J.H.; Lopez-Cabarcos, E.; López-Ruiz, B. The application of methacrylate-based polymers to enzyme biosensors. Biomol. Eng. 2006, 23, 233–245. [Google Scholar] [CrossRef]
- Adam, C.; Lacoste, J.; Lemaire, J. Photo-oxidation of polyisoprene. Polym. Degrad. Stab. 1991, 32, 51–69. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Carbone, N. Photochemical Crosslinking Reactions in Polymers. Ph.D. Thesis, Columbia University, New York, NY, USA, 2012. [Google Scholar]
- Awang, N.A.; Salleh, W.N.W.; Hasbullah, H.; Yusof, N.; Aziz, F.; Jaafar, J.; Ismail, A.F. Graft copolymerization of acrylonitrile onto recycled newspapers cellulose pulp. AIP Conf. Proc. 2017, 1885, 020244. [Google Scholar]
- Riyajan, S.A.; Sangwan, W.; Leejarkpai, T. Synthesis and properties of a novel epoxidised natural rubber-g-cassava starch polymer and its use as an impact strengthening agent. Plast. Rubber Compos. 2016, 45, 277–285. [Google Scholar] [CrossRef]
- Chen, J.S.; Ober, C.K.; Poliks, M.D.; Zhang, Y.; Wiesner, U.; Cohen, C. Controlled degradation of epoxy networks: Analysis of crosslink density and glass transition temperature changes in thermally reworkable thermosets. Polymer 2004, 45, 1939–1950. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J., Jr. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Wang, R.; Mei, H.; Ren, W.; Zhang, Y. Grafting modification of epoxidized natural rubber with poly(ethylene glycol) monomethylether carboxylic acid and ionic conductivity of graft polymer composite electrolytes. RSC Adv. 2016, 6, 107021–107028. [Google Scholar] [CrossRef]
- Woo, H.J.; Majid, S.R.; Arof, A.K. Dielectric properties and morphology of polymer electrolyte based on poly(ε-caprolactone) and ammonium thiocyanate. Mater. Chem. Phys. 2012, 134, 755–761. [Google Scholar] [CrossRef]
- Basri, N.H.; Mohamed, N.S. Conductivity Studies and Dieletric Behaviour of PVDF-HFP-PVC-LiClO4 Solid Polymer Electrolyte. Solid State Sci. Technol. 2009, 17, 63–72. [Google Scholar]
- Radhi, M.M.; Haider, A.J.; Jameel, Z.N.; Tee, T.W.; Ab Rahman, M.Z.B.; Kassim, A.B. Synthesis and characterization of grafted acrylonitrile on polystyrene modified with activated carbon using gamma-irradiation. Sci. Res. Essays 2012, 7, 790–795. [Google Scholar]
- Mas Haris, M.R.H.; Raju, G. Preparation and characterization of biopolymers comprising chitosan-grafted-ENR via acid-induced reaction of ENR50 with chitosan. Express Polym. Lett. 2014, 8, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Hamzah, R.; Abu Bakar, M.; Khairuddean, M.; Mohammed, I.A.; Adnan, R. A structural study of epoxidized natural rubber (ENR-50) and its cyclic dithiocarbonate derivative using NMR spectroscopy techniques. Molecules 2012, 17, 10974–10993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, H.; Morshedian, J.; Barikani, M.; Wagner, M.H. Correlation between molecular structure parameters and network properties of silane-grafted and moisture cross-linked polyethylenes. Adv. Polym. Technol. 2011, 30, 286–300. [Google Scholar] [CrossRef]
- Routray, C.R.; Tosh, B.; Nayak, N. Grafting of polymethyl methacrylate onto cellulose acetate in homogeneous medium using ceric (IV) ion as initiator. Indian J. Chem. Technol. 2013, 20, 202–209. [Google Scholar]
- Li, S.; Wang, Y.; Ma, L.; Zhang, X.; Dong, S.; Liu, L.; Zhou, X.; Wang, C.; Shi, Z. Synthesis of PAN with adjustable molecular weight and low polydispersity index (PDI) value via reverse atom transfer radical polymerization. Des. Monomers Polym. 2019, 22, 180–186. [Google Scholar] [CrossRef]
- Sasitaran, M.; Manroshan, S.; Lim, C.S.; Veni, B.K.; Ong, S.K.; Gunasunderi, R. Preparation and characterisation of crosslinked natural rubber (SMR CV 60) and epoxidised natural rubber (ENR-50) blends. ASEAN J. Sci. Technol. Dev. 2017, 34, 106–118. [Google Scholar] [CrossRef]
- Zhang, X.; Loo, L.S. Study of glass transition and reinforcement mechanism in polymer/layered silicate nanocomposites. Macromolecules 2009, 42, 5196–5207. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Alex, R. Compatibilization of SBR/NBR blends using chemically modified styrene-co-butadiene rubber Part 2. Effect of compatibilizer loading. Polym. Int. 2001, 50, 1298–1308. [Google Scholar] [CrossRef]
- Bashir, Z. Polyacrylonitrile, an unusual linear homopolymer with two glass transitions. Indian J. Fibre Text Res. 1999, 24, 1–9. [Google Scholar]
- Catta Preta, I.; Sakata, S.; Garcia, G.; Zimmermann, J.; Gale Beck, F.; Giovedi, C. Thermal behavior of polyacrylonitrile polymers synthesized under different conditions and comonomer compositions. J. Therm. Anal. Calorim. 2007, 87, 657–659. [Google Scholar] [CrossRef]
- Fleming, R.; Pardini, L.C.; Alves, N.; Garcia, E.; Brito, C., Jr. Synthesis and thermal behavior of polyacrylonitrile/vinylidene chloride copolymer. Polímeros 2014, 24, 259–268. [Google Scholar] [CrossRef]
- Montanheiro, T.L.D.A.; Passador, F.R.; De Oliveira, M.P.; Durán, N.; Lemes, A.P. Preparation and Characterization of Maleic Anhydride Grafted Poly (Hydroxybutirate-CO-Hydroxyvalerate)–PHBV-g-MA. Mater. Res. 2016, 19, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Qi, R.; Chen, Z.; Zhou, C. Solvothermal preparation of maleic anhydride grafted onto acrylonitrile–butadiene–styrene terpolymer (ABS). Polymer 2005, 46, 4098–4104. [Google Scholar] [CrossRef]
- Salles, V.; Bernard, S.; Brioude, A.; Cornu, D.; Miele, P. A new class of boron nitride fibers with tunable properties by combining an electrospinning process and the polymer-derived ceramics route. Nanoscale 2010, 2, 215–217. [Google Scholar] [CrossRef]
- Ahmad, Z. Polymer Dielectric Materials. In Dielectric Material; IntechOpen: London, UK, 2012. [Google Scholar]
- Sukri, N.; Mohamed, N.S.; Subban, R.H.Y. Dielectric and conduction mechanism studies of PEMA/ENR-50 blend with LiCF3SO3 salt. AIP Conf. Proc. 2017, 1877, 060002. [Google Scholar] [CrossRef] [Green Version]
- Baskaran, R.; Selvasekarapandian, S.; Hirankumar, G.; Bhuvaneswari, M.S. Dielectric and conductivity relaxations in PVAc based polymer electrolytes. Ionics 2004, 10, 129–134. [Google Scholar] [CrossRef]
- Hammami, H.; Arous, M.; Lagache, M.; Kallel, A. Study of the interfacial MWS relaxation by dielectric spectroscopy in unidirectional PZT fibres/epoxy resin composites. J. Alloys Compd. 2007, 430, 1–8. [Google Scholar] [CrossRef]
- Adohi, B.P.; Brosseau, C. Dielectric relaxation in particle-filled polymer: Influence of the filler particles and thermal treatments. J. Appl. Phys. 2009, 105, 054108. [Google Scholar] [CrossRef]
- Valverde, D.; Garcia-Bernabé, A.; Andrio, A.; García-Verdugo, E.; Luis, S.V.; Compañ, V. Free ion diffusivity and charge concentration on cross-linked polymeric ionic liquid iongel films based on sulfonated zwitterionic salts and lithium ions. Phys. Chem. Chem. Phys. 2019, 21, 17923–17932. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Sharma, A.L. Structural, electrical properties and dielectric relaxations in Na+ -ion-conducting solid polymer electrolyte. J. Phys: Condens. Mater. 2018, 30, 165402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengwa, R.J.; Dhatarwal, P.; Choudhary, S. Role of preparation methods on the structural and dielectric properties of plasticized polymer blend electrolytes: Correlation between ionic conductivity and dielectric parameters. Electrochim. Acta 2014, 142, 359–370. [Google Scholar] [CrossRef]
- Utara, S.; Boochathum, P. Effect of molecular weight of natural rubber on the compatibility and crystallization behavior of LLDPE/NR blends. Polym. Plast. Technol. Eng. 2011, 50, 1019–1026. [Google Scholar] [CrossRef]
- Samantarai, S.; Mahata, D.; Nag, A.; Nando, G.B.; Das, N.C. Functionalization of acrylonitrile butadiene rubber with meta-pentadecenyl phenol, a multifunctional additive and a renewable resource. Rubb Chem. Technol. 2017, 90, 683–698. [Google Scholar] [CrossRef]
- Dürr, C.J.; Hlalele, L.; Schneider-Baumann, M.; Kaiser, A.; Brandau, S.; Barner-Kowollik, C. Determining the Mark-Houwink parameters of nitrile rubber: A chromatographic investigation of the NBR microstructure. Polym. Chem. 2013, 4, 4755–4767. [Google Scholar] [CrossRef]
- Silva, M.J.D.; Sanches, A.O.; Malmonge, L.F.; Malmonge, J.A. Electrical, mechanical, and thermal analysis of natural rubber/polyaniline-Dbsa composite. Mater. Res. 2014, 17, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Rosniza, H.; Bakar, M.A.; Hamid, S.A.; Ismail, J. Cyclopentyl trisilanol silsesquioxanes-modified natural rubber (CpSSQ (OH)3–ENR-50) nanocomposite in the presence of tin (II) chloride dihydrate. Indones. J. Chem. 2007, 7, 111–116. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Yazdani-Pedram, M.; Brasero, J.; Lopez-Manchado, M.A. Influence of the surfactant nature on the occurrence of self-assembly between rubber particles and thermally reduced graphite oxide during the preparation of natural rubber nanocomposites. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Hussin, N.S.; Harun, F.; Chan, C.H. Thermal Properties and Conductivity of Thermally Treated Epoxidized Natural Rubber-Based Solid Polymer Electrolytes. Macromol. Symp. 2017, 376, 1700049. [Google Scholar] [CrossRef]
- Zainal, N.; Mohamed, N.S.; Idris, R. Properties of ENR-50 based electrolyte system. Sains Malays. 2013, 42, 481–485. [Google Scholar]
- Yang, D.; Ruan, M.; Huang, S.; Wu, Y.; Li, S.; Wang, H.; Shang, Y.; Li, B.; Guo, W.; Zhang, L. Improved electromechanical properties of NBR dielectric composites by poly(dopamine) and silane surface functionalized TiO2 nanoparticles. J. Mater. Chem. C 2016, 4, 7724–7734. [Google Scholar] [CrossRef]
- Yang, D.; Kong, X.; Ni, Y.; Gao, D.; Yang, B.; Zhu, Y.; Zhang, L. Novel nitrile-butadiene rubber composites with enhanced thermal conductivity and high dielectric constant. Compos. Part A Appl. Sci. Manuf. 2019, 124, 10544. [Google Scholar] [CrossRef]












| Sample ID | Mn (g mole−1) | Mw (g mole−1) | PDI (Mw/Mn) |
|---|---|---|---|
| Non-purified ENR- 25 | 990,872 | 2,460.391 | 2.48 |
| Purified ENR- 25 | 178,792 | 1,287.538 | 7.20 |
| LENR 25 (as control) | 8442 | 51,540 | 6.11 |
| * PAN (Typical) | N/A | 150,000 | N/A |
| PAN (as control) | 15,697 | 77,601 | 4.94 |
| ACN10-g-ENR1 | 50,408 | 171,064 | 3.39 |
| ACN15-g-ENR1 | 11,286 | 114,657 | 10.16 |
| ACN20-g-ENR1 | 33,547 | 252,786 | 7.54 |
| Wt Solute (W1) | Solvent (in 5 mL) | Boiling Point of Solvent | Wt of Solute after Dissolving (W2) | Solubility (%) = (W1 − W2)/W1 * 100 |
|---|---|---|---|---|
| 0.04 g | n-Hexane | 68 °C | 0.0308 g | 23% |
| 0.04 g | Xylene | 138.4 °C | 0.0089 g | 78% * |
| 0.04 g | Toluene | 110.6 °C | 0.0307 g | 23% |
| 0.04 g | Tetrahydrofuran | 66 °C | 0.0171 g | 57% |
| 0.04 g | Chloroform | 61.2 °C | 0.0230 g | 43% |
| 0.04 g | 2-butanone | 79.64 °C | 0.0124 g | 69% |
| 0.04 g | Acetone | 56 °C | 0.0292 g | 27% |
| 0.04 g | DMF | 153 °C | 0.0237 g | 41% |
| 0.04 g | Dimethyl sulfoxide | 189 °C | 0.0121 g | 70% ** |
| 0.04 g | Acetonitrile | 82 °C | 0.0302 g | 25% |
| Sample ID | Storage Modulus, E (MPa) | tan δ, Tg | ||
|---|---|---|---|---|
| At −60 °C | At 25 °C | Tan δmax | Tg °C | |
| NR [5] * | 67000 | 1.6 | 2.48 | −49 |
| ENR- 25 [5] * | 62000 | 2.1 | 2.62 | −24 |
| ENR- 50 [5] * | 3800 | 1.9 | 2.70 | −5.7 |
| NBR [46] ** | ~100 | ~ 1 | ~ 1.3 | −12 |
| ACN10-g-ENR1 | 22,286 | 713 | 0.37 | −12 |
| ACN15-g-ENR1 | 19,779 | 55 | 1.05 | −15.73 |
| ACN20-g-ENR1 | 132,353 | 56,222 | 0.34 | 13 |
| Sample ID | Tg | (50–100 °C) | (100–300 °C) | Td (300–598 °C) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tg1 | Tg2 | wt.% | Tmax. | wt.% | Tmax. | Td | wt.% | Tmax. | |
| PAN | 97 | Nd | 9.71 | Nd | 11.34 | 237 | 326 | 20.26 | 431 |
| ENR- 25 | -44 | Nd | 11.70 | 64.24 | 21.82 | Nd | 338 | 40.22 | 397 |
| ACN10-g-ENR1 | -34.96 | 102.92 | 12.68 | 65.39 | 22.23 | 202 | 346 | 41.23 | 400 |
| ACN15-g-ENR1 | -39.25 | 102.59 | 9.81 | 66.65 | 16.63 | 194 | 407 | 32.79 | 414 |
| ACN20-g-ENR1 | -34.39 | 110.73 | 12.09 | 67.79 | 14.1 | 98 | 365 | 37.28 | 406 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whba, R.; Su’ait, M.S.; Tian Khoon, L.; Ibrahim, S.; Mohamed, N.S.; Ahmad, A. Free-Radical Photopolymerization of Acrylonitrile Grafted onto Epoxidized Natural Rubber. Polymers 2021, 13, 660. https://doi.org/10.3390/polym13040660
Whba R, Su’ait MS, Tian Khoon L, Ibrahim S, Mohamed NS, Ahmad A. Free-Radical Photopolymerization of Acrylonitrile Grafted onto Epoxidized Natural Rubber. Polymers. 2021; 13(4):660. https://doi.org/10.3390/polym13040660
Chicago/Turabian StyleWhba, Rawdah, Mohd Sukor Su’ait, Lee Tian Khoon, Salmiah Ibrahim, Nor Sabirin Mohamed, and Azizan Ahmad. 2021. "Free-Radical Photopolymerization of Acrylonitrile Grafted onto Epoxidized Natural Rubber" Polymers 13, no. 4: 660. https://doi.org/10.3390/polym13040660