Melting Behavior of Heterogeneous Polymer Bulk Solids Related to Flood Fed Single Screw Extruders
Abstract
:1. Introduction
2. Methods and Experiments
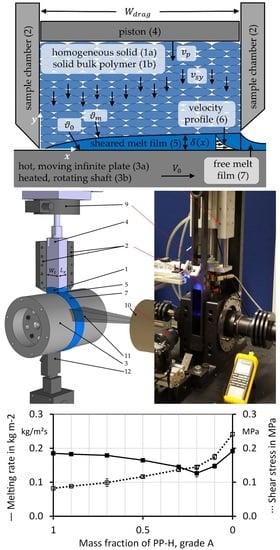
2.1. Experimental Setup for the Melting Experiments and for the Bulk Density Measurements
2.2. Melting Experiments
2.3. Bulk Density Measurements
2.4. Extrusion Experiments and Screw-Pullout Experiments
2.5. Modelling of the Melting Behavior of Pure Materials
- Calculation of the specific enthalpy needed to heat and melt the material from Equation (13).
- Assuming initial values for the mean temperature of the melt film and the mean shear rate in the melt film.
- Calculation of the viscosity of the melt film from the mean temperature and the mean shear rate according to Equations (15) and (16).
- Calculation of the thermal conductivity of the melt (if temperature related data is available).
- Calculation of the modified brinkman number from Equation (8).
- With this value, the dimensionless temperature (Equation (7)), the mean temperature of the melt film (Equation (9)) and the terms , and (Equations (12), (11) and (10)) are calculated, respectively.
- The mean shear rate in the melt film can be calculated from the velocity of the surface and from the mean melt film thickness from Equation (14).
- Now, one must go back to step 3 and apply the mean temperature of the melt film , calculated in step 6, and the mean shear rate in the melt film, calculated in step 7. These steps must be repeated till the results converge.
- The specific enthalpy needed to heat and melt the material is calculated from Equation (6).
- The melting rate per unit length is calculated from Equation (5).
2.6. Comparison of Experimental Results and Calculations
2.7. Analysis of the Melting Sequence
3. Materials
4. Results and Discussion
4.1. Bulk Density of the Material Mixtures
4.2. Melting Rates and Shear Stresses of the Pure Materials
4.3. Melting Rates and Shear Stresses of Material Polymer Mixtures
- The first mechanism assumes a complete consecutive melting of both materials, so that the material with the lower melting rate is melted after the material with the higher melting rate (Figure 6a,d). In this case, the slower melting material requires more time to melt ( than the other one (; but the melting of each material is unaffected by the other material(s).
- The second mechanism considers a complete simultaneous melting of the materials. It is supposed that different homogeneous melt films exist side by side at the same time (Figure 6b,e). If the materials melt simultaneously, the slower melting material requires a bigger proportion () of the contact area than the other one (); but the area-related melting rate of each material is again unaffected by the other material(s).
- The third mechanism also assumes simultaneous melting of the polymers. In this case, a fine structured, layered melt film consisting of alternating materials over the thickness of the melt film ( – direction) is assumed (Figure 6c,f). In this case, the melting rate of each material is affected by the other material(s).
4.4. Analysis of Extrusion Experiments and Screw-Pullout Experiments
4.5. Optical Analysis of the Melting Sequence
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campbell, G.A.; Spalding, M.A. Analyzing and Troubleshooting Single-Screw Extruders; Carl Hanser Verlag: Munich, Germany, 2013. [Google Scholar]
- Rauwendaal, C. Polymer Extrusion, 5th ed.; Carl Hanser Verlag: Munich, Germany, 2014. [Google Scholar]
- Potente, H.; Pohl, T.C. Simulation and Analyses of the Polymer-Pellet-Flow into the first Section of a Single Screw. In Proceedings of the ANTEC 2001, Dallas, TX, USA, 6–10 May 2001; pp. 185–189. [Google Scholar]
- Potente, H.; Pohl, T.C. Polymer Pellet Flow out of the Hopper into the First Section of a Single Screw. Int. Polym. Process. 2002, 17, 11–21. [Google Scholar] [CrossRef]
- Agassant, J.-F.; Avenas, P.; Carreau, P.J.; Vergnes, B.; Vincent, M. Polymer Processing: Principles and Modeling, 2nd ed.; Carl Hanser Verlag: Munich, Germany, 2017. [Google Scholar]
- Gogos, C.G.; Tadmor, Z. Principles of Polymer Processing; Wiley-Interscience: Hoboken, NJ, USA, 2014. [Google Scholar]
- White, J.L.; Potente, H. Screw Extrusion Science and Technology, 2nd ed.; Carl Hanser Verlag: Munich, Germany, 2003. [Google Scholar]
- Längauer, M.; Kneidinger, C.; Liu, K.; Zitzenbacher, G. Experimental study of the influences of the pellet shape on the bulk density and the frictional behavior of polypropylene. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Längauer, M.; Liu, K.; Kneidinger, C.; Schaffler, G.; Purgleitner, B.; Zitzenbacher, G. Experimental analysis of the influence of pellet shape on single screw extrusion. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Maddock, B.H. A Visual Analysis of Flow and Mixing in Extruder Screws. SPE J. 1959, 15, 383–389. [Google Scholar]
- Rauwendaal, C. Comparison of two melting models. Adv. Polym. Technol. 1996, 15, 135–144. [Google Scholar] [CrossRef]
- Chung, C.I. Extrusion of Polymers—Theory and Practice; Carl Hanser Verlag: Munich, Germany, 2019. [Google Scholar] [CrossRef]
- Klenk, P. Plastifiziermodelle für die Verarbeitung benetzender und nichtbenetzender Thermoplaste auf Einschnecken-Extrudern. Rheol. Acta 1968. [Google Scholar] [CrossRef]
- Gale, G.M. Dry-Blend Extrusion of Rigid PVC. Plast. Polym. 1970, 38, 183–191. [Google Scholar]
- Chung, C.I. A new look at the mechanism of melting. Plast. Eng. 1976, 32, 46–49. [Google Scholar]
- Lindt, J.T. A dynamic melting model for a single-screw extruder. Polym. Eng. Sci. 1976, 16, 284–291. [Google Scholar] [CrossRef]
- Dekker, J. Improved Screw Design for the Extrusion of Polypropylene, original title: Verbesserte Schneckenkonstruktion für das Extrudieren von Polypropylen. Kunststoffe 1976, 66, 130–135. [Google Scholar]
- Zhu, F.; Chen, L. Studies on the theory of single screw plasticating extrusion. Part I: A new experimental method for extrusion. Polym. Eng. Sci. 1991, 31, 1113–1116. [Google Scholar] [CrossRef]
- Huang, H.; Peng, Y. Theoretical modeling of dispersive melting mechanism of polymers in an extruder. Adv. Polym. Technol. 1993, 12, 343–352. [Google Scholar] [CrossRef]
- Rauwendaal, C. Finite element studies of flow and temperature evolution in single screw extruders, Plastics. Rubber Compos. 2004, 33, 390–396. [Google Scholar] [CrossRef]
- Rauwendaal, C. Dispersed Solids Melting Theory. In Proceedings of the ANTEC, New Orleans, LA, USA, 9–13 May 1993; pp. 2232–2237. [Google Scholar]
- Wilczyński, K.J.; Nastaj, A.; Wilczyński, K. A computer model for starve-fed single-screw extrusion of polymer blends. Adv. Polym. Technol. 2017, 37, 2142–2151. [Google Scholar] [CrossRef]
- Wilczyński, K.J.; Nastaj, A.; Lewandowski, A.; Wilczyński, K. Experimental and theoretical study on starve fed single screw extrusion of polymer blends. In Proceedings of the 32nd International Meeting of the Polymer Processing Society PPS-32, Lyon, France, 25–29 July 2016; 2017; p. 040004. [Google Scholar] [CrossRef] [Green Version]
- Cunha, S.M.; Gaspar-Cunha, A.; Covas, J.A. Melting of polymer blends in single-screw extrusion—An experimental study. Int. J. Mater. Form. 2009, 2, 729–732. [Google Scholar] [CrossRef] [Green Version]
- Cunha, S.M.; Gaspar-Cunha, A.; Covas, J.A. Melting of PP/PA6 Polymer Blends in Single Screw Extruders—An Experimental Study. Mater. Sci. Forum 2008, 587–588, 505–509. [Google Scholar] [CrossRef]
- Cunha, S.M.; Gaspar-Cunha, A.; Covas, J.A. Melting of Polymer Blends and Concomitant Morphology Development in Single Screw Extruders. In Proceedings of the 26th International Meeting of the Polymer Processing Society PPS-26, Banff, Canada, 4–8 July 2010; pp. 6–8. [Google Scholar]
- Wilczyński, K.; Tyszkiewicz, A.; Szymaniak, Z. Modeling for morphology development during single-screw extrusion of LDPE/PS blend. J. Mater. Process. Technol. 2001, 109, 320–323. [Google Scholar] [CrossRef]
- Wilczyński, K.; Szymaniak, Z. Modeling and experimental studies for polyblend behaviour in extrusion process. J. Cent. South Univ. Technol. 2007, 14, 369–371. [Google Scholar] [CrossRef]
- Tyagi, S.; Ghosh, A.K. Morphology development during blending of immiscible polymers in screw extruders. Polym. Eng. Sci. 2002, 42, 1309–1321. [Google Scholar] [CrossRef]
- Domingues, N.L.; Gaspar-Cunha, A.; Covas, J.A.; Manas-Zloczower, I. Computational and Experimental Study of Mixing in a Single Screw Extruder. In Proceedings of the 10th Esaform Conference on Material Forming, Zaragoza, Spain, 18–20 April 2007. [Google Scholar] [CrossRef]
- Lindt, J.T.; Ghosh, A.K. Fluid mechanics of the formation of polymer blends. Part I: Formation of lamellar structures. Polym. Eng. Sci. 1992, 32, 1802–1813. [Google Scholar] [CrossRef]
- Wilczyński, K.J.; Lewandowski, A.; Wilczyński, K. Experimental study of melting of polymer blends in a starve fed single screw extruder. Polym. Eng. Sci. 2016, 56, 1349–1356. [Google Scholar] [CrossRef]
- Domingues, N.L. Mixing in Single Screw Extrusion: Modelling and Optimization; Department of Polymer Technology, University of Minho: Braga, Portugal, 2011. [Google Scholar]
- Domingues, N.L.; Gaspar-Cunha, A.; Covas, J.A. Estimation of the morphology development of immiscible liquid-liquid systems during single screw extrusion. Polym. Eng. Sci. 2010, 50, 2194–2204. [Google Scholar] [CrossRef]
- Wilczyński, K.; Nastaj, A.; Lewandowski, A.; Wilczyński, K.J.; Buziak, K. Fundamentals of global modeling for polymer extrusion. Polymers 2019, 11, 2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilczyński, K.J.; Lewandowski, A.; Nastaj, A.; Wilczyński, K. Modeling for Starve Fed/Flood Fed Mixing Single-Screw Extruders. Int. Polym. Process. 2016, 31, 82–91. [Google Scholar] [CrossRef]
- Nastaj, A.; Wilczyński, K. Optimization for Starve Fed/Flood Fed Single Screw Extrusion of Polymeric Materials. Polymers 2020, 12, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, M. Compounding with single-screw extruders. Adv. Polym. Technol. 1997, 16, 251–262. [Google Scholar] [CrossRef]
- Strand, S.R.; Spalding, M.A.; Hyun, K.S. Modeling of the solids-conveying section of a starve-fed single-screw plasticating extruder. Plast. Eng. 1992, 48, 1–7. [Google Scholar]
- Tadmor, Z. Fundamentals of plasticating extrusion. I. A theoretical model for melting. Polym. Eng. Sci. 1966, 6, 185–190. [Google Scholar] [CrossRef]
- Kneidinger, C.; Zitzenbacher, G.; Laengauer, M.; Miethlinger, J.; Steinbichler, G. Characterizing the melting behavior of different shaped polymer bulk solids by utilizing a model experiment with an optical measuring system. In Proceedings of the ANTEC 2014, Las Vegas, NV, USA, 28–30 April 2014; pp. 1005–1012. [Google Scholar]
- Kneidinger, C.; Zitzenbacher, G.; Laengauer, M.; Miethlinger, J.; Steinbichler, G. Evaluation of the melting behavior and the melt penetration into the solid bed using a model experiment. In Proceedings of the 26th Regional Meeting of the Polymer Processing Society PPS-2016, Graz, Austria, 21–25 September 2015. [Google Scholar] [CrossRef] [Green Version]
- Shim, W. Frictional Behavior of Polymer Pellets and their Mixtures; Rensselaer Polytechnic Institute: Troy, NY, USA, 1988. [Google Scholar]
- Chung, C.I.; Hennessey, W.J.; Tusim, M.H. Frictional behavior of solid polymers on a metal surface at processing conditions. Polym. Eng. Sci. 1977, 17, 9–20. [Google Scholar] [CrossRef]
- Spalding, M.A.; Kirkpatrick, D.E.; Hyun, K.S. Coetticients ot Dynamic Friction tor Low Density Polyethylene. Polym. Eng. Sci. 1993, 33, 423–430. [Google Scholar] [CrossRef]
- Spalding, M.A.; Hyun, K.S.; Jenkins, S.R.; Kirkpatrick, D.E. Coefficients of dynamic friction and the mechanical melting mechanism for vinylidene chloride copolymers. Polym. Eng. Sci. 1995, 35, 1907–1916. [Google Scholar] [CrossRef]
- Mount, E.M., III; Watson, J.G.; Chung, C.I. Analytical melting model for extrusion: Melting rate of fully compacted solid polymers. Polym. Eng. Sci. 1982, 22, 729–737. [Google Scholar] [CrossRef]
- Mount, E.M.; Chung, C.I.; Mount, E.M.; Chung, I.C., III. Melting behavior of solid polymers on a metal surface at processing conditions. Polym. Eng. Sci. 1978, 18, 711–720. [Google Scholar] [CrossRef]
- Vermeulen, J.R.; Gerson, P.M.M.; Beek, W.J.J. The melting of a bed of polymer granules on a hot moving surface. Chem. Eng. Sci. 1971, 26, 1445–1455. [Google Scholar] [CrossRef]
- Sundstrom, D.W.; Young, C.-C. Melting rates of crystalline polymers under shear conditions. Polym. Eng. Sci. 1972, 12, 59–63. [Google Scholar] [CrossRef]
- Sundstrom, D.W.; Lo, J.R. Softening rates for polystyrene under shear conditions. Polym. Eng. Sci. 1978, 18, 422–426. [Google Scholar] [CrossRef]
- McClelland, D.E.; Chung, C.I. Shear Stress at Polymer / Metal lnterface During Melting in Extrusion. Polym. Eng. Sci. 1983, 23, 100–104. [Google Scholar] [CrossRef]
- Kim, Y.S.; Chung, C.I.; Lai, S.Y.; Hyun, K.S. Frictional Behavior of Polyethylenes with Respect to Density and Melting Characteristics. In Metallocene-Catalyzed Polymers—Materials, Properties, Processing & Markets; Benedikt, G.M., Goodall, B.L., Eds.; Plastics Design Library: Norwich, NY, USA, 1998; pp. 215–224. [Google Scholar]
- Pearson, J.R.A. On the melting of solids near a hot moving interface, with particular reference to beds of granular polymers. Int. J. Heat Mass Transf. 1976, 19, 405–411. [Google Scholar] [CrossRef]
- Bagley, E.B. End Corrections in the Capillary Flow of Polyethylene. J. Appl. Phys. 1957, 28, 624–627. [Google Scholar] [CrossRef]
- Rabinowitsch, B. Über die Viskosität und Elastizität von Solen. Z. Phys. Chem. 1929, 145A, 1–26. [Google Scholar] [CrossRef]
- Utracki, L.A.; Wilkie, C.A. Polymer Blends Handbook, 2nd ed.; Springer Science & Business Media, LLC.: Berlin, Germany, 2014. [Google Scholar]
- Scalicea, R.K.; Beckera, D.; Silveiraa, R.C. Developing a new compatibility table for design for recycling. Prod. Manag. Dev. 2009, 7, 141. [Google Scholar]
- Erhard, G. Konstruieren mit Kunststoffen; Hanser Publishers: Munich, Germany, 2008. [Google Scholar]
- Kopczynska, A. Oberflächenspannungsphänomene bei Kunststoffen—Bestimmung und Anwendung; University of Erlangen: Erlengen, Germany, 2008. [Google Scholar]
- Kneidinger, C.; Längauer, M.; Zitzenbacher, G.; Schuschnigg, S.; Miethlinger, J. Modeling and Estimation of the Pressure and Temperature dependent Bulk Density of Polymers. Int. Polym. Process. 2020, 35, 70–82. [Google Scholar] [CrossRef]
- Han, C.D. On modeling the composition dependence of the bulk viscosity of immiscible polymer blends. Polym. Eng. Sci. 2009, 49, 1671–1687. [Google Scholar] [CrossRef]
- Zitzenbacher, G.; Brunner, E. Evaluation of the influence of the tool surface on polymer melt flow using a novel rheological extrusion slit die. In Proceedings of the 27th Regional Meeting of the Polymer Processing Society PPS-2017, Dresden, Germany, 27–29 June 2017. [Google Scholar] [CrossRef]
- Zitzenbacher, G.; Huang, Z.; Holzer, C. Experimental study and modeling of wall slip of polymethylmethacrylate considering different die surfaces. Polym. Eng. Sci. 2018, 58, 1391–1398. [Google Scholar] [CrossRef]
- Zhao, R.; Macosko, C.W. Slip at polymer–polymer interfaces: Rheological measurements on coextruded multilayers. J. Rheol. 2002, 46, 145–167. [Google Scholar] [CrossRef]
- Zhang, J.; Lodge, T.P.; Macosko, C.W. Interfacial slip reduces polymer-polymer adhesion during coextrusion. J. Rheol. 2006, 50, 41–57. [Google Scholar] [CrossRef]
- Lee, P.C.; Park, H.E.; Morse, D.C.; Macosko, C.W. Polymer-polymer interfacial slip in multilayered films. J. Rheol. 2009, 53, 893–915. [Google Scholar] [CrossRef]
- Ahonguio, F.; Jossic, L.; Magnin, A. Influence of surface properties on the flow of a yield stress fluid around spheres. J. Non-Newton. Fluid Mech. 2014, 206, 57–70. [Google Scholar] [CrossRef]
- Son, Y. Investigation of interfacial slip in immiscible polymer blends. J. Polym. Res. 2011, 18, 2087–2092. [Google Scholar] [CrossRef]
- Lo, T.S.; Mihajlovic, M.; Shnidman, Y.; Li, W.; Gersappe, D. Interfacial slip in sheared polymer blends. Phys. Rev. E 2005, 72, 040801. [Google Scholar] [CrossRef] [Green Version]



















| Dimension | mm | inch | Dimension | Value |
|---|---|---|---|---|
| Screw diameter | 20.0 | 0.787 | Screw pitch | 1 D |
| Depth feeding zone | 4.0 | 0.157 | Length zone 1 1 | 8 D |
| Depth metering zone | 1.1 | 0.043 | Length zone 2 2 | 6 D |
| Axial flight width | 2.5 | 0.098 | Length zone 3 3 | 11 D |
| Radius pushing flight | 1.0 | 0.039 | Tip length | 2 D |
| Radius trailing flight | 2.5 | 0.098 | Compression Ratio | 3.64 |
| Unit | Feed throat | Zone 1 | Zone 2 | Zone 3 | Die |
|---|---|---|---|---|---|
| °C | 85 | 190 | 195 | 200 | 200 |
| °F | 185 | 374 | 383 | 392 | 392 |
| Property | PP-H, Grade A | PP-H, Grade B | PP-B | HDPE | PA6 | PMMA |
|---|---|---|---|---|---|---|
| Grade | HD 120MO | HE 125MO | BA 202E | MG 9641B | Ultramid B27E | Plexiglas 7M |
| Manufacturer | Borealis Polyolefine GmbH, Linz, Austria | Borealis Polyolefine GmbH, Linz, Austria | Borealis Polyolefine GmbH, Linz, Austria | Borealis Polyolefine GmbH, Linz, Austria | BASF SE Ludwigs-hafen, Germany | Röhm GmbH, Darmstadt Germany |
| Processing 1 | injection molding | injection molding | extrusion | injection molding | extrusion | injection molding |
| Properties 1 | good flow properties | good flow properties, | High viscosity | good flow properties | low viscosity | good flowability |
| Color | natural/ orange | blue | opaque | natural/red | opaque | transparent |
| Shape | spheroidal/ cylindrical | cylindrical | lenticular | spherical/ cylindrical | spheroidal | cylindrical |
| Property | Unit | PP-H, Grade A | PP-H, Grade B | PP-B | HDPE | PA6 | PMMA |
|---|---|---|---|---|---|---|---|
| Solid density 1 | |||||||
| Melt density 2 | |||||||
| Heat transfer coefficient of the melt 2 | |||||||
| Heat capacity of the solid 3 | |||||||
| Heat capacity of the melt 3 | |||||||
| Heat of fusion 3 λ | - | ||||||
| Consistency | |||||||
| Power law index | 0.324 | 0.376 | 0.263 | 0.486 | 0.504 | 0.239 | |
| Variation by temperature | |||||||
| Melting temperature | °C | 167.93 | 167.58 | 165 | 132 | 224 | 223 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kneidinger, C.; Schroecker, E.; Zitzenbacher, G.; Miethlinger, J. Melting Behavior of Heterogeneous Polymer Bulk Solids Related to Flood Fed Single Screw Extruders. Polymers 2020, 12, 2893. https://doi.org/10.3390/polym12122893
Kneidinger C, Schroecker E, Zitzenbacher G, Miethlinger J. Melting Behavior of Heterogeneous Polymer Bulk Solids Related to Flood Fed Single Screw Extruders. Polymers. 2020; 12(12):2893. https://doi.org/10.3390/polym12122893
Chicago/Turabian StyleKneidinger, Christian, Erik Schroecker, Gernot Zitzenbacher, and Jürgen Miethlinger. 2020. "Melting Behavior of Heterogeneous Polymer Bulk Solids Related to Flood Fed Single Screw Extruders" Polymers 12, no. 12: 2893. https://doi.org/10.3390/polym12122893