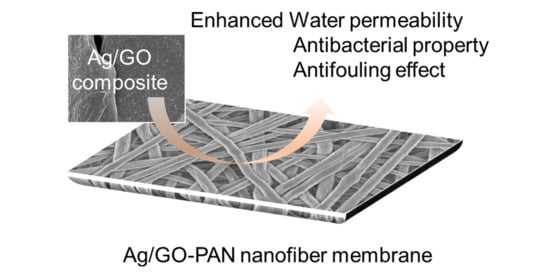
Polyacrylonitrile Nanofiber Membrane Modified with Ag/GO Composite for Water Purification System
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Ag/GO Nanocomposite
2.3. Fabrication of Ag/GO-PAN Nanofiber Membrane
2.4. Characterization
2.5. Antimicrobial Activity of PAN/Ag/GO Composite Nanofiber Membrane
2.6. Water flux and Antifouling Test of PAN/Ag/GO Composite Nanofiber Membrane
3. Results and Discussion
3.1. Structure and Morphology of Synthesized Nanoparticles and Nanofiber Membranes
3.2. Antimicrobial Activity and Water Treatment Characterization of Nanofiber Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abuzade, R.A.; Zadhoush, A.; Gharehaghaji, A.A. Air permeability of electrospun polyacrylonitrile nanoweb. J. Appl. Polym. Sci. 2012, 126, 232–243. [Google Scholar] [CrossRef]
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.; Hwang, I.-C.; Kim, K. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Persano, L.; Camposeo, A.; Pisignano, D. Active polymer nanofibers for photonics, electronics, energy generation and micromechanics. Prog. Polym. Sci. 2015, 43, 48–95. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Wang, B.; Yao, J.; Park, J. Synthesis and characterisation of hydrophilic and organophilic graphene nanosheets. Carbon 2009, 47, 1359–1364. [Google Scholar] [CrossRef]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv. Healthc. Mater. 2011, 1, 10–25. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofiber: Methods, materials, and application. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Niu, H.; Lin, T. Nanofiber manufacture, properties and applications. J. Nanomater. 2012, 2012, 725950. [Google Scholar]
- Hwang, M.; Karenson, M.O.; Elabd, Y.A. High production rate of high purity, high fidelity nafion nanofibers via needleless electrospinning. ACS Appl. Polym. Mater. 2019, 1, 2731–2740. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial upscaling of electrospinning and applications of polymer nanofibers: A review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving golbal issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Barua, B.; Saha, M.C. Investigation on jet stability, fiber diameter, and tensile properties of electrospun polyacrylonitrile nanofibrous yarns. J. Appl. Polym. Sci. 2015, 132, 41918. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Yang, K.S.; Aminabhavi, T.M. Polyacrylonitrile-based nanofibers-A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Lee, C.H.; Xie, W.; VanHouten, D.; McGrath, J.E.; Freeman, B.D.; Spano, J.; Wi, S.; Park, C.H.; Lee, Y.M. Hydrophillic silica additives for disulfonated poly(arylene ether sulfone) random copolymer membranes. J. Membr. Sci. 2012, 392–393, 157–166. [Google Scholar] [CrossRef]
- Starkova, O.; Buschhorn, S.T.; Mannov, E.; Schulte, K.; Aniskevich, A. Water transport in epoxy/MWCNT compsites. Eur. Polym. J. 2013, 49, 2138–2148. [Google Scholar] [CrossRef]
- Lee, H.D.; Kim, H.W.; Cho, Y.H.; Park, H.B. Experimental evidence of rapid water transport through carbon nanotubes embedded in polymeric desalination membranes. Small 2014, 10, 2653–2660. [Google Scholar] [CrossRef]
- Liu, L.; Xie, X.; Zambare, R.S.; Selvaraj, A.P.J.; Sowrirajalu, B.N.; Song, X.; Tang, C.Y.; Gao, C. Funtionalized graphene oxide modified polyethersulfone membrane for low-pressure anionic dye/salt fractionation. Polymers 2018, 10, 795. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Du, Y.; Feng, Q.; Huang, F.; Lu, K.; Liu, J.; Wei, Q. Nanostructures and surface nanomechanical properties of polyacrylonitrile/graphene oxide composite nanofibers by electrospinning. J. Appl. Polym. Sci. 2013, 128, 1152–1157. [Google Scholar] [CrossRef]
- Tran, C.; Kalra, V. Fabrication of porous carbon nanofibers with adjustable pore sizes as electrodes for supercapacitors. J. Power Sources 2013, 235, 289–296. [Google Scholar] [CrossRef]
- Chae, H.-R.; Lee, J.; Lee, C.-H.; Kim, I.-C.; Park, P.-K. Graphene oxide-embedded thin-film composite reverse osmosis membrane with high flux, anti-biofouling, and chlorine resistance. J. Membr. Sci. 2015, 483, 128–135. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, G.; Chu, L.; Liu, Y.; Liu, C.; Luo, S.; Wei, Y. Efficient removal of heavy metal ions with an EDTA functionalized chitosan/polyacrylamide double network hydrogel. ACS Sustain. Chem. Eng. 2017, 5, 843–851. [Google Scholar] [CrossRef]
- Pan, J.; Fei, H.; Song, S.; Yuan, F.; Yu, L. Effect of intermittent aeration on pollutants removal in subsurface wastewater infiltration system. Bioresour. Technol. 2015, 191, 327–331. [Google Scholar] [CrossRef]
- Hembach, N.; Alexander, J.; Hiller, C.; Wieland, A.; Schwartz, T. Dissemination prevention of antibiotic resistance and facultative pathogenic bacteria by ultrafiltration and ozone treatment at an urban wastewater treatment plant. Sci. Rep. 2019, 9, 12843. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; An, Y.; Li, Y.; Wong, F.S. Effect of adsoprtion/coagulation on membrane fouling in microfiltration process post-treating anaerobic digestion effluent. Desalination 2009, 242, 183–192. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MRRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Zhou, Z.; Zhang, L.; Chen, H. Improving the antifouling property of polysulfon ultrafiltration membrane by incorporation of isocyante-treated graphene oxide. Phys. Chem. Chem. Phys. 2013, 15, 9084–9092. [Google Scholar] [CrossRef]
- Yan, F.; Chen, H.; Lu, Y.; Lu, Z.; Yu, S.; Liu, M.; Gao, C. Improving the water permeability and anitfouling property of thin-film composite polyamide nanofiltration membrane by modifying the active layer with triethanolamine. J. Membr. Sci. 2016, 513, 108–116. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Khalili, S.; Mollahosseini, A.; Zirepour, A.; Rajaeian, B. Novel functionalized carbon nanotube for improving the surface properties and performance of polyethersulfon (PES) membrane. Desalination 2012, 286, 99–107. [Google Scholar] [CrossRef]
- Jang, W.; Yun, J.; Jeon, K.; Byun, H. PVdF/graphene oxide hybrid membranes via electrospinning for water treatment applications. RSC Adv. 2015, 5, 46711–46717. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, J.; Kim, J.-H.; Lee, T.; Byun, H. Electrospun PAN–GO composite nanofibers as water purification membranes. J. Appl. Polym. Sci. 2018, 135, 45858. [Google Scholar] [CrossRef]
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.L.; de Leon, A.C.C.; Rodrigues, D.F.; Advincula, R.C. On the antibacterial mechanism of graphene oxide (GO) Langmuir–Blodgett films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Chook, S.W.; Chia, C.H.; Zakaria, S.; Ayob, M.K.; Chee, K.L.; Huang, N.M.; Neoh, H.M.; Lim, H.N.; Jamal, R.; Rahman, R. Antibacterial performance of Ag nanoparticles and AgGO nanocomposites prepared via rapid microwave-assisted synthesis method. Nanoscale Res. Lett. 2012, 7, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.; Zhao, X.-R.; Jiang, X.-Y.; Teng, J.; Yu, J.-G. Hydrothermal fabrication of novel three-dimensional graphene oxide-pentaerythritol composites with abundant oxygen-containing groups as efficient adsorbents. Microchem. J. 2019, 152, 104299. [Google Scholar] [CrossRef]
- Konios, K.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef]
- Esmaeili, A.; Entezari, M.H. Facile and fast synthesis of graphene oxide nanosheets via bath ultrasonic irradication. J. Colloid Interface Sci. 2014, 432, 19–25. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Huang, L.; Li, C.; Shi, G. High-yield preparation of graphene oxide from small graphite flakes via an improved Hummers method with a simple purification process. Carbon 2015, 81, 826–834. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratio. Chem. Commun. 2001, 7, 617–618. [Google Scholar] [CrossRef]
- Jang, W.; Park, Y.; Park, C.; Seo, Y.; Kim, J.-H.; Hou, J.; Byun, H. Regulating the integrity of diverse composite nanofiber membranes using an organoclay. J. Membr. Sci. 2020, 598, 117670. [Google Scholar] [CrossRef]
- Yang, B.; Liu, Z.; Guo, Z.; Zhang, W.; Wan, M.; Qin, X.; Zhong, H. In situ green synthesis of silver-graphene oxide nanocomposites by using trytophan as a reducing and stabilizing agent and their application in SERS. Appl. Surf. Sci. 2014, 316, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Zook, J.M.; MacCuspie, R.I.; Locascio, L.E.; Halter, M.D.; Elliott, J.T. Stable nanoparticle aggregates/agglomerates of different sizes and the effect of their size on hemolytic cytotoxicity. Nanotoxicology 2011, 5, 517–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Srivastava, V.C. Simple synthesis of large graphene oxide sheets via electrochemical method coupled with oxidation process. ACS Omega 2018, 3, 10233–10242. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Z.; Zheng, Q.-B.; Qiu, H.-X.; Li, J.; Yang, J.-H. A simple method for the reduction of graphene oxide by sodium borohydride with CaCl2 as a catalyst. New Carbon Mater. 2015, 30, 41–47. [Google Scholar] [CrossRef]
- Haghi, A.K.; Akbari, M. Trends in electrospinning of natural nanofibers. Phys. Status Solidi (a) 2007, 204, 1830–1834. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical cunductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef]
- Kim, S.-G.; Park, O.-K.; Lee, J.H.; Ku, B.-C. Layer-by-layer assembled graphene oxide films and barrier properties of thermally reduced graphene oxide membranes. Carbon Lett. 2013, 14, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Thema, F.T.; Moloto, M.J.; Dikio, E.D.; Nyangiwe, N.N.; Kotsedi, L.; Maaza, M.; Khenfouch, M. Synthesis and characterization of graphene thin films by chemical reduction of exfoliated and intercalated graphite oxide. J. Chem. 2013, 2013, 150536. [Google Scholar] [CrossRef]
- Hu, M.; Zheng, S.; Mi, B. Organic fouling of graphene oxide membranes and its implications for membrane fouling control in engineered osmosis. Environ. Sci. Technol. 2016, 50, 685–693. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ng, L.Y.; Ang, W.L.; Chung, Y.T.; Rohani, R.; Mohammad, A.W. Enhancing morphology and separation performance of polyamide 6,6 membrane by minimal incorporation of silver decorated graphene oxide nanoparticles. Sci. Rep. 2019, 9, 1216. [Google Scholar] [CrossRef] [Green Version]






| Sample | Escherichia coli | Staphylococcus aureus |
|---|---|---|
| Bare-PAN | - | - |
| GO-PAN | - | - |
| Ag/GO-PAN | 1.0 ± 0.2 | 2.2 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, W.; Yun, J.; Park, Y.; Park, I.K.; Byun, H.; Lee, C.H. Polyacrylonitrile Nanofiber Membrane Modified with Ag/GO Composite for Water Purification System. Polymers 2020, 12, 2441. https://doi.org/10.3390/polym12112441
Jang W, Yun J, Park Y, Park IK, Byun H, Lee CH. Polyacrylonitrile Nanofiber Membrane Modified with Ag/GO Composite for Water Purification System. Polymers. 2020; 12(11):2441. https://doi.org/10.3390/polym12112441
Chicago/Turabian StyleJang, Wongi, Jaehan Yun, Yejun Park, In Kee Park, Hongsik Byun, and Chang Hyun Lee. 2020. "Polyacrylonitrile Nanofiber Membrane Modified with Ag/GO Composite for Water Purification System" Polymers 12, no. 11: 2441. https://doi.org/10.3390/polym12112441