The Role of Reduced Graphene Oxide in the Suspension Polymerization of Styrene and Its Effect on the Morphology and Thermal Properties of the Polystyrene/rGO Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Graphene Oxide and Reduced Graphene Oxide
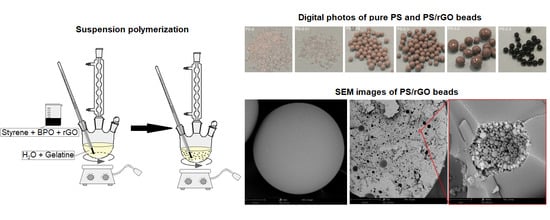
2.3. Preparation of PS/rGO Nanocomposites
2.4. Methods
3. Results and Discussion
3.1. Characterisation of Reduced Graphene Oxide
3.2. Characterisation of PS/rGO Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pötschke, P.; Arnaldo, M.H.; Radusch, H.J. Percolation behavior and mechanical properties of polycarbonate composites filled with carbon black/carbon nanotube systems. Polim. Polym. 2012, 57, 204–211. [Google Scholar] [CrossRef]
- Moaseri, E.; Karimi, M.; Baniadam, M.; Maghrebi, M. Improvements in mechanical properties of multi-walled carbon nanotube-reinforced epoxy composites through novel magnetic-assisted method for alignment of carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2014, 64, 228–233. [Google Scholar] [CrossRef]
- Nam, T.H.; Goto, K.; Yamaguchi, Y.; Premalal, E.V.A.; Shimamura, Y.; Inoue, Y.; Naito, K.; Ogihara, S. Effects of CNT diameter on mechanical properties of aligned CNT sheets and composites. Compos. Part A Appl. Sci. Manuf. 2015, 76, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Biryulin, Y.F.; Syckmanov, D.A.; Moliver, S.S.; Orlov, S.E.; Mikov, S.N.; Novoselova, A.V.; Yagovkina, M.A. Investigation of C60 fullerene films on polymer substrates. Microelectron. Eng. 2003, 69, 505–510. [Google Scholar] [CrossRef]
- Artiles, M.S.; Rout, C.S.; Fisher, T.S. Graphene-based hybrid materials and devices for biosensing. Adv. Drug Deliv. Rev. 2011, 63, 1352–1360. [Google Scholar] [CrossRef]
- Tang, L.; Yang, Z.; Duan, F.; Chen, M. Fabrication of graphene sheets/polyaniline nanofibers composite for enhanced supercapacitor properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 184–192. [Google Scholar] [CrossRef]
- Kaur, R.; Kim, K.H.; Deep, A. A convenient electrolytic assembly of graphene-MOF composite thin film and its photoanodic application. Appl. Surf. Sci. 2017, 396, 1303–1309. [Google Scholar] [CrossRef]
- Shen, B.; Li, Y.; Yi, D.; Zhai, W.; Wei, X.; Zheng, W. Strong flexible polymer/graphene composite films with 3D saw-tooth folding for enhanced and tunable electromagnetic shielding. Carbon N. Y. 2017, 113, 55–62. [Google Scholar] [CrossRef]
- Phiri, J.; Gane, P.; Maloney, T.C. General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B 2017, 215, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kobayashi, S.; Abdurrahim, M.A.; Zhang, M.J.; Khusainova, A.; Hillmyer, M.A.; Abdala, A.A.; MacOsko, C.W. Graphene/polyethylene nanocomposites: Effect of polyethylene functionalization and blending methods. Polymer 2011, 52, 1837–1846. [Google Scholar] [CrossRef]
- Verdejo, R.; Bernal, M.M.; Romasanta, L.J.; Lopez-Manchado, M.A. Graphene filled polymer nanocomposites. J. Mater. Chem. 2011, 21, 3301–3310. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, G.; Zhao, S.; Cui, J.; Gao, A.; Yan, Y. Fabrication of pristine graphene-based conductive polystyrene composites towards high performance and light-weight. Compos. Sci. Technol. 2018, 159, 232–239. [Google Scholar] [CrossRef]
- Han, Y.; Wang, T.; Gao, X.; Li, T.; Zhang, Q. Preparation of thermally reduced graphene oxide and the influence of its reduction temperature on the thermal, mechanical, flame retardant performances of PS nanocomposites. Compos. Part A Appl. Sci. Manuf. 2016, 84, 336–343. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, G.; Bai, S.; Liu, P. Preparation of halogen-free flame-retardant expandable polystyrene foam by suspension polymerization. J. Appl. Polym. Sci. 2019, 136, 47779. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Jiang, H.; Su, C.; Shao, Y.; Gao, Y.; Li, J. Preparation of recycled graphite/expanded polystyrene by a facile solvent dissolution method. J. Mater. Sci. 2019, 54, 1197–1204. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, Z.Q.; Chen, T.; Zhu, Y.; Lv, Z.H.; Gong, X.; Niu, Y.Y.; Ma, B.G. Preparation of highly dispersed expandable graphite/polystyrene composite foam via suspension polymerization non-covalently compatibilized by polystyrene with enhanced fire retardation. Carbon N. Y. 2019, 146, 503–512. [Google Scholar] [CrossRef]
- Varnagiris, S.; Girdzevicius, D.; Urbonavicius, M.; Milcius, D. Incorporation of SiO2 and TiO2 additives into expanded polystyrene foam using physical vapour deposition technique. Energy Procedia 2017, 128, 525–532. [Google Scholar] [CrossRef]
- Zhu, Z.M.; Rao, W.H.; Kang, A.H.; Liao, W.; Wang, Y.Z. Highly effective flame retarded polystyrene by synergistic effects between expandable graphite and aluminum hypophosphite. Polym. Degrad. Stab. 2018, 154, 1–9. [Google Scholar] [CrossRef]
- Braun, F.; Bellin, I.; Hahn, K. PS Foams with Low Metal Content. U.S. Patent 9,303,365, 12 April 2016. [Google Scholar]
- Wu, G.; Wang, Y.; Wang, K.; Feng, A. The effect of modified AlN on the thermal conductivity, mechanical and thermal properties of AlN/polystyrene composites. RSC Adv. 2016, 6, 102542–102548. [Google Scholar] [CrossRef]
- Yan, J.; Miao, X.; Zhang, Q.; Cui, X.; Li, J.; Wang, H. One-step preparation of black polystyrene particles via in situ suspension polymerization. Polym. Eng. Sci. 2011, 51, 294–301. [Google Scholar] [CrossRef]
- Hu, H.; Wang, X.; Wang, J.; Wan, L.; Liu, F.; Zheng, H.; Chen, R.; Xu, C. Preparation and properties of graphene nanosheets-polystyrene nanocomposites via in situ emulsion polymerization. Chem. Phys. Lett. 2010, 484, 247–253. [Google Scholar] [CrossRef]
- Dowding, P.J.; Vincent, B. Suspension polymerisation to form polymer beads. Colloids Surf. A Physicochem. Eng. Asp. 2000, 161, 259–269. [Google Scholar] [CrossRef]
- Brooks, B. Suspension polymerization processes. Chem. Eng. Technol. 2010, 33, 1737–1744. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, X.; Yang, R.; Lan, Y. Effects of triphenyl phosphate on styrene suspension polymerization process and flame retardance properties of polystyrene/triphenyl phosphate nanocomposite. Colloid Polym. Sci. 2016, 294, 1153–1163. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Chen, S.; Yang, R. Effects of polymerization conditions on particle size distribution in styrene-graphite suspension polymerization process. J. Appl. Polym. Sci. 2016, 133, 44270. [Google Scholar] [CrossRef]
- Sieradzka, M.; Fryczkowski, R.; Biniaś, D.; Biniaś, W.; Janicki, J. A facile approach to obtaining PVDF/graphene fibers and the effect of nanoadditive on the structure and properties of nanocomposites. Polym. Test. 2020, 81, 106229. [Google Scholar] [CrossRef]
- Broda, J.; Baczek, M.; Fabia, J.; Binias, D.; Fryczkowski, R. Nucleating agents based on graphene and graphene oxide for crystallization of the β-form of isotactic polypropylene. J. Mater. Sci. 2020, 55, 1436–1450. [Google Scholar] [CrossRef] [Green Version]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon N. Y. 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, Y.; Hou, Q.; Yin, R.; Liu, F.; Chen, H. Characterization of graphite oxide after heat treatment. New J. Chem. 2012, 36, 1373–1377. [Google Scholar] [CrossRef]
- Slobodian, P.; Pavlínek, V.; Lengálová, A.; Sáha, P. Polystyrene/multi-wall carbon nanotube composites prepared by suspension polymerization and their electrorheological behavior. Curr. Appl. Phys. 2009, 9, 184–188. [Google Scholar] [CrossRef]
- Chaudhary, V.; Sharma, S. Suspension polymerization technique: Parameters affecting polymer properties and application in oxidation reactions. J. Polym. Res. 2019, 26. [Google Scholar] [CrossRef]
- Erunal, E. Bead Size Distribution Dependency on Reactor Geometry and Agitation Conditions of Polystyrene Production with Suspension Polymerization. Çukurova Üniversitesi Mühendislik-Mimar. Fakültesi Derg. 2018, 33, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Li, W.; Sun, H.; Cheng, Z.; Yan, J.; Wang, H.; Cui, X. Preparation and characterization of polystyrene/modified carbon black composite beads via in situ suspension polymerization. Polym. Compos. 2013, 34, 1110–1118. [Google Scholar] [CrossRef]
- Fang, J.; Xuan, Y.; Li, Q. Preparation of polystyrene spheres in different particle sizes and assembly of the PS colloidal crystals. Sci. China Technol. Sci. 2010, 53, 3088–3093. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley&Sons Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Yin, G.; Zheng, Z.; Wang, H.; Du, Q.; Zhang, H. Preparation of graphene oxide coated polystyrene microspheres by Pickering emulsion polymerization. J. Colloid Interface Sci. 2013, 394, 192–198. [Google Scholar] [CrossRef]
- Rosa, F.; Négrier, P.; Corvis, Y.; Espeau, P. Crystal structure determination and thermal behavior upon melting of p-synephrine. Thermochim. Acta 2016, 632, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Ruan, K.; Guo, Y.; Tang, Y.; Zhang, Y.; Zhang, J.; He, M.; Kong, J.; Gu, J. Improved thermal conductivities in polystyrene nanocomposites by incorporating thermal reduced graphene oxide via electrospinning-hot press technique. Compos. Commun. 2018, 10, 68–72. [Google Scholar] [CrossRef]
- Ding, P.; Zhang, J.; Song, N.; Tang, S.; Liu, Y.; Shi, L. Growing polystyrene chains from the surface of graphene layers via RAFT polymerization and the influence on their thermal properties. Compos. Part A Appl. Sci. Manuf. 2015, 69, 186–194. [Google Scholar] [CrossRef]
- Basu, S.; Singhi, M.; Satapathy, B.K.; Fahim, M. Dielectric, electrical, and rheological characterization of graphene-filled polystyrene nanocomposites. Polym. Compos. 2013, 34, 2082–2093. [Google Scholar] [CrossRef]
- Lian, F.; Xing, B.; Zhu, L. Comparative study on composition, structure, and adsorption behaviorof activated carbons derived from different synthetic waste polymers. Colloid Interface Sci. 2011, 360, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Levchik, S.V.; Weil, E.D. A review on thermal decomposition and combustion ofthermoplastic polyesters. Polym. Adv. Technol. 2004, 15, 691–700. [Google Scholar] [CrossRef]







| Samples | PS-0 | PS-0.01 | PS-0.05 | PS-0.1 | PS-0.2 | – | PS-0.3 | |
|---|---|---|---|---|---|---|---|---|
| Water Phase | DW, g | 170 | 170 | 170 | 170 | 170 | 170 | 250 |
| Gel, g | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 6.3 | |
| Oil Phase | St, g | 45.4 | 45.4 | 45.4 | 45.4 | 45.4 | 45.4 | 18.0 |
| PS, g | 0 | 0 | 0 | 0 | 0 | 0 | 5.1 | |
| BPO, % | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.5 | |
| rGO, % | 0 | 0.01 | 0.05 | 0.1 | 0.2 | 0.3 | 0.3 |
| Sample | Tg1, °C | Tg2, °C | TER, °C | ∆HER, J/g |
|---|---|---|---|---|
| PS-0 | 84.7 | 93.7 | 103.2 | 1.77 |
| PS-0.01 | 86.5 | 97.1 | 102.1 | 1.73 |
| PS-0.05 | 78.7 | 95.5 | 101.3 | 1.36 |
| PS-0.1 | 75.8 | 93.8 | 100.6 | 0.69 |
| PS-0.2 | – | 91.1 | 98.9 | 0.56 |
| PS-0.3 | – | 91.3 | 98.7 | 0.17 |
| Sample | Weight Loss Up to Temperature 30 °C | Weight Loss in Range of 30–175 °C | Weight Loss in Range of 175–470 °C | Residue after Heating Up to 470 °C |
|---|---|---|---|---|
| Δm1, % | Δm2, % | Δm3, % | R, % | |
| PS-0 | 0.02 | 0.28 | 99.70 | – |
| PS-0.01 | 0.02 | 0.33 | 99.65 | – |
| PS-0.05 | 0.47 | 4.21 | 94.97 | 0.35 |
| PS-0.1 | 1.47 | 4.95 | 93.22 | 0.36 |
| PS-0.2 | 0.64 | 2.67 | 96.28 | 0,41 |
| PS-0.3 | 0.01 | 10.65 * | 88.30 | 1.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sieradzka, M.; Fabia, J.; Biniaś, D.; Fryczkowski, R.; Janicki, J. The Role of Reduced Graphene Oxide in the Suspension Polymerization of Styrene and Its Effect on the Morphology and Thermal Properties of the Polystyrene/rGO Nanocomposites. Polymers 2020, 12, 1468. https://doi.org/10.3390/polym12071468
Sieradzka M, Fabia J, Biniaś D, Fryczkowski R, Janicki J. The Role of Reduced Graphene Oxide in the Suspension Polymerization of Styrene and Its Effect on the Morphology and Thermal Properties of the Polystyrene/rGO Nanocomposites. Polymers. 2020; 12(7):1468. https://doi.org/10.3390/polym12071468
Chicago/Turabian StyleSieradzka, Marta, Janusz Fabia, Dorota Biniaś, Ryszard Fryczkowski, and Jarosław Janicki. 2020. "The Role of Reduced Graphene Oxide in the Suspension Polymerization of Styrene and Its Effect on the Morphology and Thermal Properties of the Polystyrene/rGO Nanocomposites" Polymers 12, no. 7: 1468. https://doi.org/10.3390/polym12071468