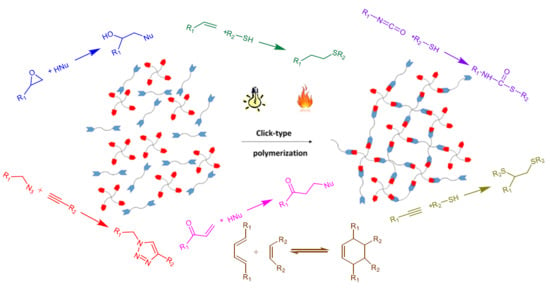
The Use of Click-Type Reactions in the Preparation of Thermosets
Abstract
:1. Introduction
2. Phenomenological and Theoretical Treatment of Click Reactions in the Preparation of Thermosets
2.1. Network Build-Up Analysis
- (a)
- Unequal reactivity of the groups in the monomer, i.e., by substitution effects. Negative substitution effects are common in reactive systems based on epoxy reactions with aromatic amines [28], or aza-Michael addition with aliphatic amines [29,30], in which the reactivity of a secondary amine hydrogen decreases significantly after the reaction of a primary amine. The effect is to delay branching and gelation. This effect has been studied in detail in the literature using different methodologies [10,31,32]. The effect of negative substitution effects in terms of gel point conversion may be difficult to determine because differences may fall within the experimental error range, but it may be much easier to determine it in terms of the critical ratio at gelation [10]. In general, it can be stated that would increase in the presence of an excess monomer with a negative subsitution effect. Conversely, would decrease in the presence of an excess of a monomer with a negative substitution effect.
- (b)
- Intramolecular cyclization reactions take place when there is a nonnegligible probability that a reactive group meets a reactive group from the same molecule. In consequence, the reaction takes place without an increase in molecular weight or branching, thereby delaying gelation. The presence of cyclic structures may lead to the presence of network defects such as dangling chains and a soluble fraction at the end of the reaction, and to an effective reduction in crosslinking density and equilibrium modulus [31,33,34,35], and this is further aggravated in diluted systems because of the reduced probability of intermolecular reactions. Some reactive systems have an intrinsic tendency to form intramolecular cycles due to the particular flexibility of some of the reactants [31,32,33,36]. Some approximations to the determination of the extent of cyclization based on the spanning-tree concept have been described in the literature [30,35] and references therein. Parameters such as or have a fairly linear dependence with the inverse of the concentration of reactive groups [31,33], thereby making it possible to determine the ring-free (i.e., no cyclization) parameters by extrapolating .
- (c)
- The formation of preferred nonrandom structures, i.e., driven by thermodynamics, is also described in the literature, such as silanol condensation or cyclotrimerization reactions [10,37]. A combination of structural fragments becomes nonrandom, and therefore, larger preferred structures are formed. This may be taken into consideration using a suitable kinetic-structural model, so that these larger structures can be incorporated into the recursive model.
2.2. Glass Transition-Conversion Relationships
2.3. Temperature-Transformation Diagrams
3. Preparation of Thermosets by Nucleophilic Attack to Epoxy Resins
4. Preparation of Thermosets by Michael Addition
5. Preparation of Thermosets by Addition of Thiol Monomers on Unsaturated Compounds
6. Poly(thiourethane) Networks by Thiol-Isocyanate Reaction
7. Preparation of Thermosets by Azide-Yne Polymerization
8. Preparation of Thermosets by Diels-Alder Cycloaddition
9. Combination of Two or More Click Reaction: Dual Curing
10. Advanced Technological Applications of Thermosets Obtained by Means of Click Methodologies
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Qin, A.; Lam, J.W.Y.; Tang, B.Z. Click polymerization: Progresses, challenges, and opportunities. Macromolecules 2010, 43, 8693–8702. [Google Scholar] [CrossRef]
- Lowe, A.B.; Bowman, C.N. Thiol-X Chemistries in Polymer and Materials Science; RSC Publishing: Cambridge, UK, 2013. [Google Scholar]
- Binder, W.H.; Sachsenhofer, R. ‘Click’ chemistry in polymer and material science: An update. Macromol. Chem. Rapid Commun. 2008, 29, 952–981. [Google Scholar] [CrossRef]
- Espeel, P.; Du Prez, F.E. “Click”—Inspired chemistry in macromolecular science: Matching recent progress and user expectations. Macromolecules 2015, 48, 2–14. [Google Scholar] [CrossRef]
- Tunca, U. Orthogonal multiple click reactions in synthetic polymer chemistry. J. Polym. Sci. Part. A: Polym. Chem. 2014, 52, 3147–3165. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Kiskan, B.; Yagci, Y. Externally stimulated click reactions for macromolecular syntheses. Prog. Polym. Sci. 2016, 52, 19–78. [Google Scholar] [CrossRef]
- Qin, A.; Tang, B.Z. Click Polymerization; Polymer Chemistry Series N° 30; RSC Publishing: Cambridge, UK, 2018. [Google Scholar]
- Hoyle, C.E.; Lowe, A.B.; Bowman, C.N. Thiol-click chemistry: A multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 2010, 39, 1355–1387. [Google Scholar] [CrossRef]
- Pascault, J.P.; Sautereau, H.; Verdu, J.; Williams, R.J.J. Thermosetting Polymers; Marcel Dekker, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Ramis, X.; Fernández-Francos, X.; De la Flor, S.; Ferrando, F.; Serra, A. Click-based dual curing thermosets and their applications. In Thermosets. Structure, Properties and Applications, 2nd ed.; Guo, Q., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 511–541. [Google Scholar]
- Turi, E.A. Thermal Characterization of Polymeric Materials, 2nd ed.; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Dušek, K.; Dušková-Smrčková, M. Network structure formation during crosslinking of organic coating systems. Prog. Polym Sci. 2000, 25, 1215–1260. [Google Scholar] [CrossRef]
- Hale, A.; Macosko, C.W.; Bair, H.E. Glass transition temperature as a function of conversion in thermosetting polymers. Macromolecules 1991, 24, 2610–2621. [Google Scholar] [CrossRef]
- Aronhime, M.T.; Gillham, J.K. Time-Temperature-Transformation (TTT) Cure Diagram of Thermosetting Polymeric Systems; Springer: Berlin, Germany, 1986; pp. 83–113. [Google Scholar]
- Lory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Miller, D.R.; Macosko, C.W. A New Derivation of post gel properties of network polymers. Macromolecules 1976, 9, 206–211. [Google Scholar] [CrossRef]
- Miller, D.R.; Valles, E.M.; Macosko, C.W. Calculation of molecular parameters for stepwise polyfunctional polymerization. Polym. Eng. Sci. 1979, 19, 272–283. [Google Scholar] [CrossRef]
- Dušek, K.; Dušková-Smrčková, M.; Fedderly, J.J.; Lee, G.F.; Lee, J.D.; Hartmann, B. Polyurethane networks with controlled architecture of dangling chains. Macromol. Chem. Phys. 2002, 203, 1936–1948. [Google Scholar] [CrossRef]
- Dušek, K.; Dušková-Smrčková, M. Modeling of polymer network formation from preformed precursors. Macromol. React. Eng. 2012, 6, 426–445. [Google Scholar] [CrossRef]
- Fernández-Francos, X.; Ramis, X. Structural analysis of the curing of epoxy thermosets crosslinked with hyperbranched poly(ethyleneimine)s. Eur. Polym. J. 2015, 70, 286–305. [Google Scholar] [CrossRef] [Green Version]
- Voit, B. New developments in hyperbranched polymers. J. Polym. Sci. Part. A Polym. Chem. 2000, 38, 2505–2525. [Google Scholar] [CrossRef]
- Van Benthem, R.A.T.M.; Meijerink, N.; Geladé, E.; de Koster, C.G.; Muscat, D.; Froehling, P.E.; Hendriks, P.H.M.; Vermeulen, C.J.A.A.; Zwartkruis, T.J.G. Synthesis and characterization of bis(2-hydroxypropyl)amide-based hyperbranched polyesteramides. Macromolecules 2001, 34, 3559–3566. [Google Scholar] [CrossRef]
- Nair, D.P.; Cramer, N.B.; Gaipa, J.C.; McBride, M.K.; Matherly, E.M.; McLeod, R.R.; Shandas, R.; Bowman, C.N. Two-stage reactive polymer network forming systems. Adv. Funct. Mater. 2012, 22, 1502–1510. [Google Scholar] [CrossRef]
- Fernández-Francos, X.; Konuray, A.O.; Belmonte, A.; De la Flor, S.; Serra, À.; Ramis, X. Sequential curing of off-stoichiometric thiol–epoxy thermosets with a custom-tailored structure. Polym. Chem. 2016, 7, 2280–2290. [Google Scholar] [CrossRef] [Green Version]
- Saharil, F.; Forsberg, F.; Liu, Y.; Bettotti, P.; Kumar, N.; Haraldsson, F.N.; der Wijngaart, W.V.; Gylfason, K.B. Dry adhesive bonding of nanoporous inorganic membranes to microfluidic devices using the OSTE(+) dual-cure polymer. J. Micromech. Microeng. 2013, 23, 025021. [Google Scholar] [CrossRef] [Green Version]
- Russo, C.; Serra, À.; Fernández-Francos, X.; De la Flor, S. Characterization of sequential dual-curing of thiol-acrylate-epoxy systems with controlled thermal properties. Eur. Polym. J. 2019, 112, 376–388. [Google Scholar] [CrossRef]
- Girard-Reydet, E.; Riccardi, C.C.; Sautereau, H.; Pascault, J.P. Epoxy-Aromatic Diamine Kinetics. Part 1. Modeling and Influence of the Diamine Structure. Macromolecules 1995, 28, 7599–7607. [Google Scholar] [CrossRef]
- Mather, B.D.; Viswanathan, K.; Miller, K.M.; Long, T.E. Michael addition reactions in macromolecular design for emerging technologies. Prog. Polym. Sci. 2006, 31, 487–531. [Google Scholar] [CrossRef]
- Retailleau, M.; Ibrahim, A.; Croutxé-Barghorn, C.; Allonas, X.; Ley, C.; Le Nouen, D. One-pot three-step polymerization system using double click Michael addition and radical photopolymerization. ACS Macro Lett. 2015, 4, 1327–1331. [Google Scholar] [CrossRef]
- Matejka, L. Amine cured epoxide networks: Formation, structure, and properties. Macromolecules 2000, 33, 3611–3619. [Google Scholar] [CrossRef]
- Tanaka, Y.; Stanford, J.L.; Stepto, R. Interpretation of gel points of an epoxy-amine system including ring formation and unequal reactivity: Reaction scheme and gel-point prediction. Macromolecules 2012, 45, 7186–7196. [Google Scholar] [CrossRef]
- Dušková-Smrčková, M.; Valentová, H.; Ďuračková, A.; Dušek, K. Effect of dilution on structure and properties of polyurethane networks. Pregel and postgel cyclization and phase separation. Macromolecules 2010, 43, 6450–6462. [Google Scholar] [CrossRef]
- Pereda, S.; Brandolin, A.; Vallés, E.M.; Sarmoria, C. Copolymerization between A3 and B2 with ring formation and different intrinsic reactivity in one of the monomers. Macromolecules 2001, 34, 4390–4400. [Google Scholar] [CrossRef]
- Sarmoria, C.; Miller, D.R. Spanning-tree models for Af homopolymerizations with intramolecular reactions. Comput. Theor. Polym. Sci. 2001, 11, 113–127. [Google Scholar] [CrossRef]
- Belmonte, A.; Fernández-Francos, X.; Serra, À.; De la Flor, S. Phenomenological characterization of sequential dual-curing of off-stoichiometric “thiol-epoxy” systems: Towards applicability. Mater. Des. 2017, 113, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Kasehagen, L.J.; Rankin, S.E.; McCormick, A.V.; Macosko, C.W. Modeling of first shell substitution effects and preferred cyclization in sol-gel polymerization. Macromolecules 1997, 30, 3921–3929. [Google Scholar] [CrossRef]
- Karkanas, P.I.; Partridge, I.K. Cure modeling and monitoring of epoxy/amine resin systems. II. Network formation and chemoviscosity modeling. J. App. Polym. Sci. 2000, 77, 2178–2188. [Google Scholar] [CrossRef]
- Cadenato, A.; Salla, J.M.; Ramis, X.; Morancho, J.M.; Marroyo, L.M.; Martin, J.L. Determination of gel and vitrification times of thermoset curing process by means of TMA, DMTA and DSC techniques TTT diagram. J. Therm. Anal. 1997, 49, 269–279. [Google Scholar] [CrossRef]
- Ramis, X.; Salla, J.M. Time-temperature transformation (TTT) cure diagram of an unsaturated polyester resin. J. Polym. Sci. Part. B Polym. Phys. 1997, 35, 371–388. [Google Scholar] [CrossRef]
- Verchere, D.; Sautereau, H.; Pascault, J.P.; Riccardi, C.C.; Moschiar, S.M.; Williams, R.J.J. Buildup of epoxycycloaliphatic amine networks. Kinetics, vitrification, and gelation. Macromolecules 1990, 23, 725–731. [Google Scholar] [CrossRef]
- Venditti, R.A.; Gillham, J.K. Relationship between the glass transition temperature (Tg) and fractional conversion for thermosetting systems. J. App. Polym. Sci. 1997, 64, 3–14. [Google Scholar] [CrossRef]
- Pascault, J.P.; Williams, R.J.J. Glass transition temperature versus conversion relationships for thermosetting polymers. J. Polym. Sci. Part. B Polym. Phys. 1990, 28, 85–95. [Google Scholar] [CrossRef]
- Couchman, P.R. Compositional variation of glass-transition temperatures. 2. Application of the thermodynamic theory to compatible polymer blends. Macromolecules 1978, 11, 1156–1161. [Google Scholar] [CrossRef]
- Belmonte, A.; Däbritz, F.; Ramis, X.; Serra, A.; Voit, B.; Fernández-Francos, X. Cure kinetics modeling and thermomechanical properties of cycloaliphatic epoxy-anhydride thermosets modified with hyperstar polymers. J. Polym. Sci. Part. B Polym. Phys. 2014, 52, 1227–1242. [Google Scholar] [CrossRef]
- Santiago, D.; Fernàndez-Francos, X.; Ramis, X.; Salla, J.M.; Sangermano, M. Comparative curing kinetics and thermal-mechanical properties of DGEBA thermosets cured with a hyperbranched poly(ethyleneimine) and an aliphatic triamine. Thermochim. Acta 2011, 526, 9–21. [Google Scholar] [CrossRef]
- Antonucci, V.; Giordano, M.; Nicolais, L. Effect of the segmental mobility restriction on the thermoset chemical kinetics. J. Polym. Sci. Part. A Polym. Chem. 2002, 40, 3757–3770. [Google Scholar] [CrossRef]
- Teil, H.; Page, S.A.; Michaud, V.; Manson, J.A.E. TTT-cure diagram of an anhydride-cured epoxy system including gelation, vitrification, curing kinetics model, and monitoring of the glass transition temperature. J. App. Polym. Sci. 2004, 93, 1774–1787. [Google Scholar] [CrossRef]
- Konuray, O.; Salla, J.M.; Morancho, J.M.; Fernández-Francos, X.; García-Alvarez, M.; Ramis, X. Time-temperature-transformation (TTT) diagram of dual-curable epoxy thermosets obtained via two sequential epoxy-amine condensations. Thermochim. Acta 2019, 678, 178305. [Google Scholar] [CrossRef]
- Pascault, J.P.; Williams, R.J.J. Epoxy Polymers. New Materials and Innovations; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Lee, H.; Neville, K. Handbook of Epoxy Resins; McGraw Hill: New York, NY, USA, 1982. [Google Scholar]
- Kang, T.; Amir, R.J.; Khan, A.; Ohshimizu, K.; Hunt, J.N.; Sivanandan, K.; Montañez, M.I.; Malkoch, M.; Ueda, M.; Hawker, C.J. Facile access to internally functionalized dendrimers through efficient and orthogonal click reactions. Chem. Commun. 2010, 46, 1556–1558. [Google Scholar] [CrossRef]
- Smith, I.T. The mechanism of the crosslinking of epoxide resins by amines. Polymer 1961, 2, 95–108. [Google Scholar] [CrossRef]
- Matějka, L.; Dušek, K.; Dobáš, I. Curing of epoxy resins with amines. Polym. Bull. 1985, 14, 309–315. [Google Scholar]
- Ignatenko, V.Y.; Ilyin, S.O.; Kostyuk, A.V.; Bondarenko, G.N.; Antonov, S.V. Acceleration of epoxy resin curing by using a combination of aliphatic and aromatic amines. Polym. Bull. 2020, 77, 1519–1540. [Google Scholar] [CrossRef]
- Grillet, A.C.; Galy, J.; Pascault, J.P.; Bardin, I. Effects of the structure of the aromatic curing agent on the cure kinetics of epoxy networks. Polymer 1989, 30, 2094–2103. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C.C. Sustainable lignin-based epoxy resins cured with aromatic and aliphatic amine curing agents: Curing kinetics and thermal properties. Thermochim. Acta 2015, 618, 48–55. [Google Scholar] [CrossRef]
- Hollande, L.; Do Marcolino, I.; Balaguer, P.; Domenek, S.; Gross, R.A.; Allais, F. Preparation of renewable. Epoxy-amine resins with tunable thermo-mechanical properties, wettability and degradation abilities from lignocellulose- and plant oils-derived components. Front. Chem. 2019, 7, 159. [Google Scholar] [CrossRef]
- Liu, Z.S.; Erhan, S.Z.; Calvert, P.D. Solid freeform fabrication of epoxidized soybean oil/epoxy composites with di-, tri-, and polyethylene amine curing agents. J. Appl. Polym. Sci. 2004, 93, 356–363. [Google Scholar] [CrossRef]
- Wan, J.; Gan, B.; Li, C.; Molina-Aldareguia, J.; Li, Z.; Wang, X.; Wang, D.-Y. A novel biobased epoxy resin with high mechanical stiffness and low flammability: Synthesis, characterization and properties. J. Mater. Chem. A 2015, 3, 21907–21921. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a promising biobased building-block for monomer synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Qi, Y.; Weng, Z.; Zhang, K.; Wang, J.; Zhang, S.; Liu, C.; Jian, X. Magnolol-based bio-epoxy resin with acceptable glass transition temperature, processability and flame retardancy. Chem. Eng. J. 2020, 387, 124115. [Google Scholar] [CrossRef]
- Morancho, J.M.; Ramis, X.; Fernández-Francos, X.; Salla, J.M.; Konuray, A.O.; Serra, A. Curing of off-stoichiometric amine–epoxy thermosets. J. Therm. Anal. Calorim. 2018, 133, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Konuray, O.; Areny, N.; Morancho, J.M.; Fernández-Francos, X.; Serra, À.; Ramis, X. Preparation and characterization of dual-curable off-stoichiometric amine-epoxy thermosets with latent reactivity. Polymer 2018, 146, 42–52. [Google Scholar] [CrossRef]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; Serra, A. New catalyst for diglycidylether of bisphenol a curing based on thiol-epoxy click reaction. Eur. Polym. J. 2014, 59, 377–386. [Google Scholar] [CrossRef]
- Sangermano, M.; Vitale, A.; Dietliker, K. Photolatent amines producing a strong base as photocatalyst for the in-situ preparation of organic–inorganic hybrid coatings. Polymer 2014, 55, 1628–1635. [Google Scholar] [CrossRef]
- Guzmán, D.; Mateu, B.; Fernández-Francos, X.; Ramis, X.; Serra, À. Novel thermal curing of cycloaliphatic resins by thiol-epoxy click process with several multifunctional thiols. Polym. Int. 2017, 66, 1697–1707. [Google Scholar] [CrossRef]
- Kasetaite, S.; De la Flor, S.; Serra, A.; Ostrauskaite, J. Effect of selected thiols on cross-linking of acrylated epoxidized soybean oil and properties of resulting polymers. Polymers 2018, 10, 439. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.C.; Rong, M.Z.; Zhang, M.Q.; Chen, J.; Yang, G.C.; Li, X.M. Self-healing polymeric materials using epoxy/mercaptan as the healant. Macromolecules 2008, 41, 5197–5202. [Google Scholar] [CrossRef]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; De la Flor, S.; Serra, A. Preparation of new biobased coatings from a triglycidyl eugenol derivative through thiol-epoxy click reaction. Prog. Org. Coat. 2018, 114, 259–267. [Google Scholar] [CrossRef]
- Guzmán, D.; Serra, A.; Ramis, X.; Fernández-Francos, X.; De la Flor, S. Fully renewable thermosets based on bis-eugenol prepared by thiol-click chemistry. React. Funct. Polym. 2019, 136, 153–166. [Google Scholar] [CrossRef]
- Guzmán, D.; Santiago, D.; Serra, A.; Ferrando, F. Novel bio-based epoxy thermosets based on triglycidyl phloroglucinol prepared by thiol-epoxy reaction. Polymers 2020, 12, 337. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Hu, Y.; Man, L.; Yuan, T.; Zhang, C.; Yang, Z. Biobased thiol-epoxy shape memory networks from gallic acid and vegetable oils. Eur. Polym. J. 2019, 112, 619–628. [Google Scholar] [CrossRef]
- Koc, O.P.; Acar, S.B.; Uyar, T.; Tasdelen, M.A. In situ preparation of thermoset/clay nanocomposites via thiol-epoxy click chemistry. Polym. Bull. 2018, 75, 4901–4911. [Google Scholar]
- Hutchinson, J.M.; Roman, F.; Folch, A. Epoxy-thiol systems filled with boron nitride for high thermal conductivity applications. Polymers 2018, 10, 340. [Google Scholar] [CrossRef] [Green Version]
- Isarn, I.; Ramis, X.; Ferrando, F.; Serra, A. Thermoconductive Thermosetting Composites Based on Boron Nitride Fillers and Thiol-Epoxy Matrices. Polymers 2018, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Belmonte, A.; Lama, G.C.; Gentile, G.; Cerruti, P.; Ambrogi, V.; Fernández-Francos, X.; De la Flor, S. Thermally-triggered free-standing shape-memory actuators. Eur. Polym. J. 2017, 97, 241–252. [Google Scholar] [CrossRef]
- Nair, D.P.; Podgórski, M.; Chantani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The thiol-Michael addition click reaction: A powerful and widely used tool in materials chemistry. Chem. Mat. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Chatani, S.; Wang, C.; Podgórski, M.; Bowman, C.N. Triple shape memory materials incorporating two distinct polymer networks formed by selective thiol–Michael addition reactions. Macromolecules 2014, 47, 4949–4954. [Google Scholar] [CrossRef]
- Konuray, O.; Fernández-Francos, X.; Ramis, X.; Serra, A. Acetoacetate based thermosets prepared by dual-Michael addition reactions. Polymers 2019, 11, 1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, G.; Fernández-Francos, X.; Serra, À.; Sangermano, M.; Ramis, X. Environmentally-friendly processing of thermosets by two-stage sequential aza-Michael addition and free-radical polymerization of amine-acrylate mixtures. Polym. Chem. 2015, 6, 6987–6997. [Google Scholar] [CrossRef] [Green Version]
- Konuray, A.O.; Ruiz, A.; Morancho, J.M.; Salla, J.M.; Fernández-Francos, X.; Serra, À.; Ramis, X. Sequential dual curing by selective Michael addition and free radical polymerization of acetoacetate-acrylate-methacrylate mixtures. Eur. Polym. J. 2018, 98, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Xi, W.; Peng, H.; Aguirre-Soto, A.; Kloxin, C.J.; Stansbury, J.W.; Bowman, C.N. Spatial and temporal control of thiol-Michael addition via photocaged superbase in photopatterning and two-stage polymer networks formation. Macromolecules 2014, 47, 6159–6165. [Google Scholar] [CrossRef] [Green Version]
- Konuray, O.; Fernández-Francos, X.; Ramis, X.; Serra, A. State of the art in dual-curing acrylate systems. Polymers 2018, 10, 178. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.W.; Hoyle, C.E.; Lowe, A.B.; Bowman, M. Nucleophile-initiated thiol-Michael reactions: Effect of organocatalyst, thiol, and ene. Macromolecules 2010, 43, 6381–6388. [Google Scholar] [CrossRef]
- Xi, W.; Wang, C.; Kloxin, C.J.; Bowman, C.N. Nitrogen-centered nucleophile catalyzed thiol-vinylsulfone addition, another thiol-ene “click” reaction. ACS Macro Lett. 2012, 1, 811–814. [Google Scholar] [CrossRef]
- Podgórski, M.; Chatani, S.; Bowman, C.N. Development of glassy step-growth thiol-vinyl sulfone polymer networks. Macromol. Rap. Commun. 2014, 35, 1497–1502. [Google Scholar] [CrossRef] [Green Version]
- Chatani, S.; Nair, D.P.; Bowman, C.N. Relative reactivity and selectivity of vinyl sulfones and acrylates towards the thiol–Michael addition reaction and polymerization. Polym. Chem. 2013, 4, 1048–1055. [Google Scholar] [CrossRef]
- Parker, S.; Reit, R.; Abitz, H.; Ellson, G.; Yang, K.; Lund, B.; Voit, W.E. High-Tg thiol-click thermoset networks via the thiol-maleimide Michael addition. Macromol. Rapid Commun. 2016, 37, 1027–1032. [Google Scholar] [CrossRef]
- Shibata, M.; Hashimoto, Y. High performance thermosetting bismaleimide resins via thiol-maleimide “click” reaction. Eur. Polym. J. 2017, 93, 561–571. [Google Scholar] [CrossRef]
- Konuray, O.; Fernández-Francos, X.; Ramis, X.; Serra, A. New allyl-functional catalytic comonomers for sequential thiol-Michael and radical thiol-ene reactions. Polymer 2018, 138, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Moszner, N.; Rheinberger, V. Reaction behaviour of monomeric β-ketoesters, 4. Polymer network formation by Michael reaction of multifunctional acetoacetates with multifunctional acrylates. Macromol. Rapid Commun. 1995, 16, 135–138. [Google Scholar] [CrossRef]
- Pavlinec, J.; Moszner, N. Photocured polymer networks based on multifunctional beta-ketoesters and acrylates. J. Appl. Polym. Sci. 1997, 65, 165–178. [Google Scholar] [CrossRef]
- Konuray, O.; Liendo, F.; Fernández-Francos, X.; Serra, A.; Sangermano, M.; Ramis, X. Sequential curing of thiol-acetoacetate-acrylate thermosets by latent Michael addition reactions. Polymer 2017, 113, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Liu, Y.; He, C.; Chung, T.; Goh, S. Effects of chemistries of trifunctional amines on mechanisms of Michael addition polymerizations with diacrylates. Macromolecules 2004, 37, 6763–6770. [Google Scholar] [CrossRef]
- Konuray, A.O.; Fernández-Francos, X.; Serra, À.; Ramis, X. Sequential curing of amine-acrylate-methacrylate mixtures based on selective aza-Michael addition followed by radical photopolymerization. Eur. Polym. J. 2016, 84, 256–267. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Z.; Guo, B. Regulation of mechanical properties of diene rubber cured by oxa-Michael reaction via manipulating network structure. Polymer 2018, 144, 57–64. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol-enes: Chemistry of the past with promise for the future. J. Polym. Sci. Part. A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Chandrasekaran, S. Click Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Uygun, M.; Tasdelen, M.A.; Yagci, Y. Influence of type of initiation on thiol-ene “click” chemistry. Macromol. Chem. Phys. 2010, 211, 103–110. [Google Scholar] [CrossRef]
- Moszner, N.; Schoeb, W.; Rheinberger, V. Synthesis, characterization and thiol-ene polymerization of hydrolyzed/condensed norbornenyl silic acid ester. Polym. Bull. 1996, 37, 289–295. [Google Scholar] [CrossRef]
- Resetco, C.; Hendriks, B.; Badiaband, N.; Du Prez, F. Thiol–Ene chemistry for polymer coatings and surface modification – building in sustainability and performance. Mater. Horiz. 2017, 4, 1041–1053. [Google Scholar] [CrossRef]
- Goethals, F.; Frank, D.; Du Prez, F. Protected thiol strategies in macromolecular design. Prog. Polym. Sci. 2017, 64, 76–113. [Google Scholar] [CrossRef]
- Weems, A.C.; Delle Chiaie, K.R.; Worch, J.C.; Stubbs, C.J.; Dove, A.P. Terpene- and terpenoid-based polymeric resins for stereolithography 3D printing. Polym. Chem. 2019, 10, 5959–5966. [Google Scholar] [CrossRef] [Green Version]
- Jawerth, M.E.; Brett, C.J.; Terrier, C.; Larsson, P.T.; Lawoko, M.; Roth, S.V.; Lundmark, S.; Johansson, M. Mechanical and morphological properties of lignin-based thermosets. ACS Appl. Polym. Mater. 2020, 2, 668–676. [Google Scholar] [CrossRef]
- Jawerth, M.; Lawoko, M.; Lundmark, S.; Perez-Berumen, C.; Johansson, M. Allylation of a lignin model phenol: A highly selective reaction under benign conditions towards a new thermoset resin platform. RSC Adv. 2016, 6, 96281–96288. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Sun, L.; Ou, R.; Fan, Q.; Li, L.; Guo, C.; Liu, Z.; Wang, Q. Flame retardant eugenol-based thiol-ene polymer networks with high mechanical strength and transparency. Chem. Eng. J. 2019, 368, 359–368. [Google Scholar] [CrossRef]
- Cheng, C.; Li, J.; Yang, F.; Li, Y.; Hu, Z.; Wang, J. Renewable eugenol-based functional polymers with self-healing and high temperature resistance properties. J. Polym. Res. 2018, 25, 57. [Google Scholar] [CrossRef]
- Larsen, D.B.; Sønderbæk-Jørgensen, R.; Duus, J.Ø.; Daugaard, A.E. Investigation of curing rates of bio-based thiol-ene films from diallyl 2, 5-furandicaboxylate. Eur. Polym. J. 2018, 102, 1–8. [Google Scholar] [CrossRef]
- Yokose, R.; Shimasaki, T.; Teramoto, N.; Shibata, M. Amino acid-incorporated polymer network by thiol-ene polymerization. Express Polym. Lett. 2015, 9, 744–755. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Q.; Wang, K.; Ke, M.; Hu, Y.; Shen, L.; Geng, Q.; Cheng, J. From biomass resources to functional materials: A fluorescent thermosetting material based on resveratrol via thiol-ene click chemistry. Eur. Polym. J. 2020, 123, 109416. [Google Scholar] [CrossRef]
- Colucci, G.; Aluigi, A.; Tonin, C.; Bongiovanni, R. Photopolymerization of keratin-based thiol-ene coatings. Prog.Org. Coat. 2014, 77, 1104–1110. [Google Scholar] [CrossRef]
- McNair, O.D.; Janisse, A.P.; Krzeminski, D.E.; Brent, D.E.; Gould, T.E.; Rawlins, J.W.; Savin, D.A. Impact properties of thiol−ene networks. ACS Appl. Mater. Interfaces 2013, 5, 11004–11013. [Google Scholar] [CrossRef] [PubMed]
- McNair, O.D.; Sparks, B.J.; Janisse, A.P.; Brent, D.P.; Patton, D.L.; Savin, D.A. Highly tunable thiol−ene networks via dual thiol addition. Macromolecules 2013, 46, 5614–5621. [Google Scholar] [CrossRef]
- Schreck, K.M.; Leung, D.; Bowman, C.N. Hybrid organic/inorganic thiol-ene-based photopolymerized networks. Macromolecules 2011, 44, 7520–7529. [Google Scholar] [CrossRef] [Green Version]
- Trey, S.M.; Nilsson, C.; Malmström, E.; Johansson, M. Thiol-ene networks and reactive surfaces via photoinduced polymerization of allyl ether functional hyperbranched polymers. Prog. Org. Coat. 2010, 67, 348–355. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-yne ‘click’/coupling chemistry and recent applications in polymer and materials synthesis and modification. Polymer 2014, 55, 5517–5549. [Google Scholar] [CrossRef]
- Fairbanks, D.B.; Scott, T.F.; Kloxin, C.J.; Anseth, K.S.; Bowman, C.N. Thiol-yne photopolymerizations: Novel mechanism, kinetics, and step-growth formation of highly cross-linked networks. Macromolecules 2009, 42, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yu, B.; Matsushima, H.; Hoyle, C.E.; Lowe, A.B. The thiol-isocyanate click reaction: Facile and quantitative access to ω-end-functional poly (N,N-diethylacrylamide) synthesized by RAFT radical polymerization. Macromolecules 2009, 42, 6537–6542. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.P.; Boutevin, B.; Ganachaud, F. On the versatility of urethane/urea bonds: Reversibility, blocked isocyanate, and non-isocyanate polyurethanes. Chem. Rev. 2012, 113, 80–118. [Google Scholar] [CrossRef]
- Kuypers, S.; Paramanik, S.K.; D’Olieslaeger, L.; Reekmans, G.; Peters, M.; D’Haen, J.; Vanderzande, D.; Junkers, T.; Adriaensens, P.; Ethirajan, A. Interfacial thiol–isocyanate reactions for functional nanocarriers: A facile route towards tunable morphologies and hydrophilic payload encapsulation. Chem. Commun. 2015, 51, 15858–15861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatani, S.; Sheridan, R.; Podgorski, M.; Nair, D.P.; Bowman, C.N. Temporal control of thiol-click chemistry. Chem. Mater. 2013, 25, 3897–3901. [Google Scholar] [CrossRef]
- Salmi, H.; Allonas, X.; Ley, C. Polythiourethane networks catalyzed by photobase generators. Prog. Org. Coat. 2016, 100, 81–85. [Google Scholar] [CrossRef]
- Gamardella, F.; Ramis, X.; De la Flor, S.; Serra, A. Preparation of poly(thiourethane) thermosets by controlled thiol-isocyanate click reaction using a latent organocatalyst. React. Funct. Polym. 2019, 134, 174–182. [Google Scholar] [CrossRef]
- Hillewaere, X.K.D.; Teixeira, R.F.A.; Nguyen, L.-T.T.; Ramos, J.A.; Rahier, H.; Du Prez, F.E. Autonomous self-healing of epoxy thermosets with thiol-isocyanate chemistry. Adv. Funct. Mater. 2014, 24, 5575–5583. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, B.; Jin, J.; Li, J. High refractive index polythiourethane networks with high mechanical property via thiol-isocyanate click reaction. Polymer 2019, 180, 121746. [Google Scholar] [CrossRef]
- Jaffrennou, B.; Droger, N.; Mechin, F.; Halary, J.L.; Pascault, J.P. Characterization structural transitions and properties of a tightly crosslinked polythiourethane network for optical applications. e-Polymers 2005, 5, 81. [Google Scholar] [CrossRef]
- Yan, J.; Ariyasivam, S.; Weerasinghe, D.; He, J.; Chisholm, B.; Chen, Z.; Webster, D. Thermoset coatings from bio-based thiols. Polym. Int. 2012, 61, 602–608. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Torkelson, J.M. Reprocessable polymer networks via thiourethane dynamic chemistry: Recovery of cross-link density after recycling and proof of principle solvolysis leading to monomer recovery. Macromolecules 2019, 52, 8207–8216. [Google Scholar] [CrossRef]
- Gamardella, F.; Guerrero, F.; De la Flor, S.; Ramis, X.; Serra, A. A new class of vitrimers based on aliphatic poly (thiourethane) networks with shape memory and permanent shape reconfiguration. Eur. Polym. J. 2019, 122, 109361. [Google Scholar] [CrossRef]
- Gamardella, F.; De la Flor, S.; Ramis, X.; Serra, A. Recyclable poly (thiourethane) vitrimers with high Tg. Influence of the isocyanate structure. React. Funct. Polym. 2020, 151, 104574. [Google Scholar] [CrossRef]
- Huisgen, R. 1, 3-Dipolar cycloadditions. Past and future. Angew. Chem. Int. Ed. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Baskin, J.M.; Bertozzi, C.R. Copper-Free Click Chemistry in Click Chemistry for Biotechnology and Materials Science; Lahann, J., Ed.; Wiley: Chichester, UK, 2009. [Google Scholar]
- Pascoal, M.; Brook, M.A.; Gonzaga, F.; Zepeda-Velazquez, L. Thermally controlled silicone functionalization using selective Huisgen reactions. Eur. Polym. J. 2015, 69, 429–437. [Google Scholar] [CrossRef]
- Ozdogan, R.; Daglar, O.; Durmaz, H.; Tasdelen, M.A. Aliphatic polyester/polyhedral oligomeric silsesquioxanes hybrid networks via copper-free 1,3-dipolar cycloaddition click reaction. J. Polym. Sci. Part. A Polym. Chem. 2019, 57, 2222–2227. [Google Scholar] [CrossRef]
- Arslan, I.; Tasdelen, M.A. POSS-based hybrid thermosets via photoinduced copper-catalyzed azide–alkyne cycloaddition click chemistry. Des. Monomers Polym. 2016, 19, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen cycloaddition process: Copper (I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper (I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- McBride, M.K.; Gong, T.; Nair, D.P.; Bowman, C.N. Photo-mediated copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) “click” reactions for forming polymer networks as shape memory materials. Polymer 2014, 55, 5880–5884. [Google Scholar] [CrossRef] [Green Version]
- Reshmi, S.K.; Vijayalakshmi, K.P.; Thomas, D.; Arunan, E.; Nair, C.P.R. Glycidyl azide polymer crosslinked through triazoles by click chemistry: Curing, mechanical and thermal properties. Propellants Explos. Pyrotech. 2013, 38, 525–532. [Google Scholar] [CrossRef]
- Adzima, B.J.; Tao, Y.; Kloxin, C.J.; DeForest, C.A.; Anseth, K.S.; Bowman, C.N. Spatial and temporal control of the alkyne–azide cycloaddition by photoinitiated Cu(II) reduction. Nature Chem. 2011, 3, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Tasdelen, M.A.; Yagci, Y. Light-induced copper (I)-catalyzed click chemistry. Tetrahedron Lett. 2010, 51, 6945–6947. [Google Scholar] [CrossRef]
- Gong, T.; Adzima, B.J.; Baker, N.H.; Bowman, C.N. Photopolymerization reactions using the photoinitiated copper (I)-catalyzed azide-alkyne cycloaddition (CUAAC) reaction. Adv. Mater. 2013, 25, 2024–2028. [Google Scholar] [CrossRef] [PubMed]
- Uysal, N.; Acik, G.; Tasdelen, M.A. Soybean oil based thermoset networks via photoinduced CuAAC click chemistry. Polym. Bull. 2017, 66, 999–1004. [Google Scholar] [CrossRef]
- Song, H.B.; Baranek, A.; Worrell, B.T.; Cook, W.D.; Bowman, C.N. Photopolymerized triazole-based glassy polymer networks with superior tensile toughness. Adv. Funct. Mater. 2018, 28, 1801095. [Google Scholar] [CrossRef]
- Oz, E.; Uyar, T.; Esen, H.; Tasdelen, M.A. Simultaneous photoinduced electron transfer and photoinduced CuAAC processes for antibacterial thermosets. Prog. Org. Coat. 2017, 105, 252–257. [Google Scholar] [CrossRef]
- Sunitha, K.; Kumar, K.S.S.; Mathew, D.; Nair, C.P.R. Shape memory polymers (SMPs) derived from phenolic cross-linked epoxy resin via click chemistry. Mater. Lett. 2013, 99, 101–104. [Google Scholar] [CrossRef]
- Potzsch, R.; Voit, B. Thermal and photochemical crosslinking of hyperbranched polyphenylene with organic azides. Macromol. Rapid Commun. 2012, 33, 635–639. [Google Scholar] [CrossRef]
- Díaz, D.D.; Punna, S.; Holzer, P.; McPherson, A.K.; Sharpless, K.B.; Fokin, V.V.; Finn, M.G. Click chemistry in materials synthesis. 1. Adhesive polymers from copper-catalyzed azide-alkyne cycloaddition. J. Polym. Sci. Part. A Polym. Chem. 2004, 42, 4392–4403. [Google Scholar] [CrossRef]
- Le Baut, N.; Díaz, D.D.; Punna, S.; Finn, M.G.; Brown, H.R. Study of high glass transition temperature thermosets made from the copper(I)-catalyzed azide-alkyne cycloaddition reaction. Polymer 2007, 48, 239–244. [Google Scholar] [CrossRef]
- Wan, L.; Huang, F.; Du, L. New heat-resistant polytriazole adhesives: Investigation of adhesion of polytriazole resins to metals. J. Adhes. Sci. Technol. 2013, 27, 1767–1777. [Google Scholar]
- Pratama, P.A.; Peterson, A.M.; Palmese, G.R. The role of maleimide structure in the healing of furan-functionalized epoxy–amine thermosets. Polym. Chem. 2013, 4, 5000–5006. [Google Scholar] [CrossRef]
- Post, W.; Susa, A.; Blaauw, R.; Molenveld, K.; Knoop, R.J.I. A review on the potential and limitations of recyclable thermosets for structural applications. Polym. Rev. 2019. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C. Degradable thermosets based on labile bonds or linkages: A review. Prog. Polym. Sci. 2018, 76, 65–110. [Google Scholar] [CrossRef]
- Stevens, M.P.; Jenkins, A.D. Crosslinking of polystyrene via pendant maleimide groups. J. Polym. Sci. Part. A Polym. Chem. 1979, 17, 3675–3685. [Google Scholar] [CrossRef]
- Tian, Q.Y.; Yuan, Y.C.; Rong, M.Z.; Zhang, M.Q. A thermally remendable epoxy resin. J. Mater. Chem. 2009, 19, 1289–1296. [Google Scholar] [CrossRef]
- Dello Iacono, S.; Martone, A.; Pastore, A.; Filippone, G.; Acierno, D.; Zarrelli, M.; Giordano, M.; Amendola, E. Thermally activated multiple self-healing Diels-Alder epoxy system. Polym. Eng. Sci. 2017, 57, 674–679. [Google Scholar] [CrossRef]
- Truong, T.T.; Nguyen, H.T.; Phan, M.N.; Nguyen, L.-T.T. Study of Diels–Alder reactions between furan and maleimide model compounds and the preparation of a healable thermo-reversible polyurethane. J. Polym. Sci. Part. A Polym. Chem. 2018, 56, 1806–1814. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Hsieh, C.-Y.; Chen, Y.-W. Thermally reversible cross-linked polyamides and thermo-responsive gels by means of Diels–Alder reaction. Polymer 2006, 47, 2581–2586. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Ishida, H. Polymerization of an AB-type benzoxazine monomer toward different polybenzoxazine networks: When Diels−Alder reaction meets benzoxazine chemistry in a single-component resin. Macromolecules 2019, 52, 7386–7395. [Google Scholar] [CrossRef]
- Trovatti, E.; Lacerda, T.M.; Carvalho, A.J.F.; Gandini, A. Recycling tires? Reversible crosslinking of poly (butadiene). Adv. Mater. 2015, 27, 2242–2245. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally remendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wudl, F.; Mal, A.K.; Shen, H.; Nutt, S.R. New thermally remendable highly cross-linked polymeric materials. Macromolecules 2003, 36, 1802–1807. [Google Scholar] [CrossRef]
- Canadell, J.; Fischer, H.; De With, G.; Van Benthem, R.A.T.M. Stereoisomeric effects in thermo-remendable polymer networks based on Diels–Alder crosslink reactions. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 3456–3467. [Google Scholar] [CrossRef]
- Zeng, C.; Seino, H.; Ren, J.; Hatanaka, K.; Yoshie, N. Self-healing bio-based furan polymers cross-linked with various bis-maleimides. Polymer 2013, 54, 5351–5357. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Bowman, C.N. Covalent adaptable networks: Smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.M.; Van Assche, G.; Maurer, F.H.J.; Van Mele, B. Thermophysical characterization of a reversible dynamic polymer network based on kinetics and equilibrium of an amorphous furan maleimide Diels-Alder cycloaddition. Polymer 2017, 120, 176–188. [Google Scholar] [CrossRef]
- Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Dynamic covalent polymer networks: From old chemistry to modern day innovations. Adv. Mater. 2017, 29, 1606100. [Google Scholar] [CrossRef]
- Gandini, A. The furan/maleimide Diels-Alder reaction: A versatile click-unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Gandini, A.; Armando, J.D.; Silvestre; Dora, C. Reversible click chemistry at the service of macromolecular materials. Part 1: Kinetics of the Diels−Alder reaction applied to furan-maleimide model compounds and linear polymerizations. Eur. Polym. J. 2008, 44, 4029–4036. [Google Scholar] [CrossRef]
- Lacerda, T.M.; Gandini, A. Marriage of furans and vegetable oils through click chemistry for the preparation of macromolecular materials: A succinct review. J. Renew. Mater. 2014, 2, 1–12. [Google Scholar] [CrossRef]
- Toncelli, C.; Bouwhuis, S.; Broekhuis, A.A.; Picchioni, F. Cyclopentadiene-functionalized polyketone as self-cross-linking thermo-reversible thermoset with increased softening temperature. J. Apply. Polym. Chem. 2016, 42924. [Google Scholar] [CrossRef]
- Defize, T.; Riva, R.; Jerome, C.; Alexandre, M. Multifunctional poly (epsilon-caprolactone)-forming networks by Diels-Alder cycloaddition: Effect of the adduct on the shape-memory properties. Macromol. Chem. Phys. 2012, 213, 187–197. [Google Scholar] [CrossRef]
- Kim, T.-D.; Luo, J.; Tian, Y.; Ka, J.-W.; Tucker, N.M.; Haller, M.; Kang, J.-W.; Jen, A.K.-Y. Diels-Alder “Click Chemistry” for highly efficient electrooptic polymers. Macromolecules 2006, 39, 1676–1680. [Google Scholar] [CrossRef]
- Luo, K.J.; Huang, L.B.; Wang, Y.; Yu, J.R.; Zhu, J.; Hu, Z.M. Tailoring the properties of Diels-Alder reaction crosslinked high-performance thermosets by different bismaleimides. Chin. J. Polym. Sci. 2020, 38, 268–277. [Google Scholar] [CrossRef]
- Okhay, N.; Mignard, N.; Jegat, C.; Taha, M. Diels–Alder thermoresponsive networks based on high maleimide-functionalized urethane prepolymers. Des. Monomers Polym. 2013, 16, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Billiet, S.; Bruycker, K.D.; Driessen, F.; Goosens, H.; Van Speybroeck, V.; Winne, J.M.; Du Prez, F.E. Triazolinediones enable ultrafast and reversible click chemistry for the design of dynamic polymer systems. Nature Chem. 2014, 6, 815–821. [Google Scholar] [CrossRef]
- Li, J.H.; Zhang, G.P.; Deng, L.B.; Jiang, K.; Zhao, S.F.; Gao, Y.J.; Sun, R.; Wong, C.P. Thermally reversible and self-healing novolac epoxy resins based on Diels-Alder chemistry. J. Appl. Polym. Sci. 2015, 132, 42167. [Google Scholar] [CrossRef]
- Schäfer, S.; Kickelbick, G. Double reversible networks: Improvement of self-healing in hybrid materials via combination of Diels−Alder cross-linking and hydrogen bonds. Macromolecules 2018, 51, 6099–6110. [Google Scholar] [CrossRef]
- Buonerba, A.; Speranza, V.; Capacchione, C.; Milione, S.; Grassi, A. Improvement of tensile properties, self-healing and recycle of thermoset styrene/2-vinylfuran copolymers via thermal triggered rearrangement of covalent crosslink. Eur. Polym. J. 2018, 99, 368–377. [Google Scholar] [CrossRef]
- Zhang, Y.; Broekhuis, A.A.; Picchioni, F. Thermally self-healing polymeric materials: The next step to recycling thermoset polymers? Macromolecules 2009, 42, 1906–1912. [Google Scholar] [CrossRef] [Green Version]
- Amato, D.N.; Strange, G.A.; Swanson, J.P.; Chavez, A.D.; Roy, S.E.; Varney, K.L.; Machado, C.A.; Amato, D.V.; Costanzo, P.J. Synthesis and evaluation of thermally-responsive coatings based upon Diels–Alder chemistry and renewable materials. Polym. Chem. 2014, 5, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Li, Z.; Wang, B.; Liu, F.; Liu, Y.; Liu, F. Recyclable biobased materials based on Diels-Alder cycloaddition. J. Appl. Polym. Sci. 2019, 47352. [Google Scholar] [CrossRef]
- Duval, A.; Couture, G.; Caillol, S.; Avérous, L. Biobased and aromatic reversible thermoset networks from condensed tannins via the Diels−Alder reaction. ACS Sustain. Chem. Eng. 2017, 5, 1199–1207. [Google Scholar] [CrossRef]
- Kirchhoff, R.A.; Bruza, K.J. Polymers from Benzocyclobutenes. Adv. Polym. Sci. 1994, 117, 1–66. [Google Scholar]
- Yang, J.; Cheng, Y.; Xiao, F. Synthesis, thermal and mechanical properties of benzocyclobutene-functionalized siloxane thermosets with different geometric structures. Eur. Polym. J. 2012, 48, 751–760. [Google Scholar] [CrossRef]
- Tan, L.S.; Arnold, F.; Soloski, E.J. Benzocyclobutene in polymer synthesis.3. Heat-resistant thermosets based on Diels-Alder polymerization of a bisbenzocyclobutene and a bismaleimide. J. Polym. Sci. Part. A Polym. Chem. 1988, 26, 3103–3117. [Google Scholar] [CrossRef]
- Chan, J.W.; Hoyle, C.E.; Lowe, A.B. Sequential phosphine-catalyzed, nucleophilic thiol-ene/radical-mediated thiol-yne reactions and the facile orthogonal synthesis of polyfunctional materials. J. Am. Chem. Soc. 2009, 131, 5751–5753. [Google Scholar] [CrossRef]
- Shin, J.; Matsushima, H.; Comer, C.M.; Bowman, C.N.; Hoyle, C.E. Thiol-isocyanate-ene ternary networks by sequential and simultaneous thiol click reactions. Chem. Mater. 2010, 22, 2616–2625. [Google Scholar] [CrossRef]
- Matsushima, H.; Shin, J.; Bowman, C.N.; Hoyle, C.E. Thiol-isocyanate-acrylate ternary networks by selective thiol-click chemistry. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 3255–3264. [Google Scholar] [CrossRef]
- Gamardella, F.; Sabatini, V.; Ramis, X.; Serra, À. Tailor-made thermosets obtained by sequential dual-curing combining isocyanate-thiol and epoxy-thiol click reactions. Polymer 2019, 174, 200–209. [Google Scholar] [CrossRef]
- Perrot, D.; Croutxé-Barghorn, C.; Allonas, X. UV-curable thio-ether-urethane network with tunable properties. J. Polym. Sci. Part. A Polym. Chem. 2016, 54, 3119–3126. [Google Scholar] [CrossRef]
- Peng, H.; Nair, D.P.; Kowalski, B.A.; Xi, W.; Gong, T.; Wang, C.; Cole, M.; Cramer, N.B.; Xie, X.; McLeod, R.R.; et al. High performance graded rainbow holograms via two-stage sequential orthogonal thiol-click chemistry. Macromolecules 2014, 47, 2306–2315. [Google Scholar] [CrossRef]
- Peng, H.; Wang, C.; Xi, W.; Kowalski, B.A.; Gong, T.; Xie, X.; Wang, W.; Nair, D.P.; McLeod, R.R.; Bowman, C.N. Facile image patterning via sequential thiol-Michael/thiol-yne click reactions. Chem. Mater. 2014, 26, 6819–6826. [Google Scholar] [CrossRef]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; Serra, A. Preparation of click thiol-ene/thiol-epoxy thermosets by controlled photo/thermal dual curing sequence. RSC Adv. 2015, 5, 101623–101633. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; De la Flor, S.; Serra, A. New bio-based materials obtained by thiol-ene/thiol-epoxy dual curing click procedures from eugenol derivates. Eur. Polym. J. 2017, 93, 530–544. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Zhu, L.; Wang, S.; Yang, L.; Song, Z.; Liu, X.; Zhu, J. UV-thermal dual cured anti-bacterial thiol-ene networks with superior performance from renewable resources. Polymer 2017, 108, 215–222. [Google Scholar] [CrossRef]
- Shibata, M.; Sugane, K.; Satoh, A. Photo-thermally cured eugenol-derived epoxy resins by simultaneous thiol-ene/thiol-epoxy/thiol-maleimide triple “click” reactions. J. Polym. Res. 2018, 25, 234. [Google Scholar] [CrossRef]
- Acebo, C.; Fernández-Francos, X.; Ramis, X.; Serra, À. Multifunctional allyl-terminated hyperbranched poly(ethyleneimine) as component of new thiol-ene/thiol-epoxy materials. React. Funct. Polym. 2016, 99, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Badia, J.D.; Teruel-Juanes, R.; Acebo, C.; Gil-Castell, O.; Serra, A.; Ribes-Greus, A. Dielectric spectroscopy of novel thiol-ene/epoxy thermosets obtained from allyl-modified hyperbranched poly(ethyleneimine) and diglycidylether of bisphenol A. Eur. Polym. J. 2019, 113, 98–106. [Google Scholar] [CrossRef]
- Acebo, C.; Fernández-Francos, X.; Ramis, X.; Serra, À. Thiol-yne/thiol-epoxy hybrid crosslinked materials based on propargyl modified hyperbranched poly(ethyleneimine) and diglycidylether of bisphenol A resins. RSC Adv. 2016, 6, 61576–61584. [Google Scholar] [CrossRef] [Green Version]
- Flores, M.; Tomuta, A.M.; Fernández-Francos, X.; Ramis, X.; Sangermano, M.; Serra, A. A new two-stage curing system: Thiol-ene/epoxy homopolymerization using an allyl terminated hyperbranched polyester as reactive modifier. Polymer 2013, 54, 5473–5481. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Puente Urbina, B.A.; Cabello Valdez, L.V.; Berlanga Duarte, L.; Guerrero Santos, R.; García Valdez, A.E.; Soucek, M.D. Effect of introducing a cationic system into a thiol-ene photopolymerizable formulation. J. Polym. Sci. Part. A Polym. Chem. 2007, 45, 4829–4843. [Google Scholar] [CrossRef]
- Sangermano, M.; Cerrone, M.; Colucci, G.; Roppolo, I.; Ortiz, R.A. Preparation and characterization of hybrid thiol-ene/epoxy UV-thermal dual-cured systems. Polym. Int. 2010, 59, 1046–1051. [Google Scholar] [CrossRef]
- Jin, K.; Wilmot, N.; Heath, W.H.; Torkelson, J.M. Phase-separated thiol-epoxy-acrylate hybrid polymer networks with controlled cross-link density synthesized by simultaneous thiol-acrylate and thiol-epoxy click reactions. Macromolecules 2016, 49, 4115–4123. [Google Scholar] [CrossRef]
- Konuray, A.O.; Fernández-Francos, X.; Ramis, X. Curing kinetics and characterization of dual-curable thiol-acrylate-epoxy thermosets with latent reactivity. React. Funct. Polym. 2018, 122, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Brown, H.R.; Hawker, C.J. One-pot fabrication of robust interpenetrating hydrogels via orthogonal click reactions. J. Polym. Sci. Part. A Polym. Chem. 2016, 54, 1459–1467. [Google Scholar] [CrossRef]
- Konuray, O.; García, A.; Morancho, J.M.; Fernández-Francos, X.; Serra, À.; Ferrando, F.; García-Alvarez, M.; Ramis, X. Hard epoxy thermosets obtained via two sequential epoxy-amine condensations. Eur. Polym. J. 2019, 116, 222–231. [Google Scholar] [CrossRef]
- Ma, X.; Xie, H.; Shi, W. A highly branched polythiourethane acrylate used for UV-curable high antireflection coatings. Prog. Org. Coat. 2013, 76, 870–875. [Google Scholar] [CrossRef]
- Gan, Y.; Jiang, X.; Yin, J. Thiol–ene photo-curable hybrid silicone resin for LED encapsulation: Enhancement of light extraction efficiency by facile self-keeping hemisphere coating. J. Mater. Chem. C 2014, 2, 5533–5539. [Google Scholar] [CrossRef]
- Northrop, B.H.; Frayne, S.H.; Choudhary, U. Thiol-maleimide “click” chemistry: Evaluating the influence of solvent, initiator, and thiol on the reaction mechanism, kinetics and selectivity. Polym. Chem. 2015, 6, 3415–3430. [Google Scholar] [CrossRef]
- Reit, R.; Abitz, H.; Reddy, N.; Parker, S.; Wei, A.; Aragon, N.; Ho, M.; Weittenhiller, A.; Kang, T.; Eckerd, M.; et al. Thiol–epoxy/maleimide ternary networks as softening substrates for flexible electronics. J. Mater. Chem. B 2016, 4, 5367–5374. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Ware, T.; Marcotte, R.; Lund, B.R.; Smith, D.W., Jr.; Di Prima, M.; Rennaker, R.L.; Voit, W. A comparison of polymer substrates for photolithographic processing of flexible bioelectronics. Biomed. Microdevices 2013, 15, 925–939. [Google Scholar] [CrossRef]
- Khire, V.S.; Yi, Y.; Clark, N.A.; Bowman, C.N. Formation and surface modification of nanopatterned thiol-ene substrates using step and flash imprint lithography. Adv. Mater. 2008, 20, 3308–3313. [Google Scholar] [CrossRef]
- Lyon, G.B.; Cox, L.W.; Goodrich, J.T.; Baranek, A.D.; Ding, Y.; Bowman, C.N. Remoldable thiol–ene vitrimers for photopatterning and nanoimprint lithography. Macromolecules 2016, 49, 8905–8913. [Google Scholar] [CrossRef]
- Cox, L.M.; Martinez, A.M.; Blevins, A.K.; Sowan, N.; Ding, Y.; Bowman, C.N. Nanoimprint lithography: Emergent materials and methods of actuation. Nano Today 2020, 100838. [Google Scholar] [CrossRef]
- Carlborg, C.F.; Haraldsson, T.; Öberg, K.; Malkoch, M.; Van Der Wijngaart, W. Beyond PDMS: Off-stoichiometry thiol-ene (OSTE) based soft lithography for rapid prototyping of microfluidic devices. Lab. Chip 2011, 11, 3136–3147. [Google Scholar] [CrossRef] [Green Version]
- Sticker, D.; Geczy, R.; HÄfeli, U.O.; Kutter, J.P. Thiol−ene based polymers as versatile materials for microfluidic devices for life sciences applications. ACS Appl. Mater. Interfaces 2020, 12, 10080–10095. [Google Scholar] [CrossRef]
- Upadhyaya, J.; Bounds, C.O.; Totaro, N.; Thakuri, S.; Garber, L.; Vincent, M.; Huang, Z.; Pojman, J.A. Production and analysis of stable microfluidic devices with tunable surface hydrophilicity via the in-situ tertiary-amine catalyzed Michael addition of a multifunctional thiol to a multifunctional acrylate. Eur. Polym. J. 2020, 126, 109482. [Google Scholar] [CrossRef]
- Yeo, H.; Khan, A. Photoinduced proton-transfer polymerization: A practical synthetic tool for soft lithography applications. J. Am. Chem. Soc. 2020, 142, 3479–3488. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, D.G.; Yeo, H.; Rao, J.; Zhu, Z.; Shin, J.; Jeong, K.; Kim, S.; Jung, H.W.; Khan, A. Proton transfer hydrogels: Versatility and applications. J. Am. Chem. Soc. 2018, 140, 6700–6709. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, Q.; Wei, G.; Liu, R.; Li, Z. Highly stable thiol-ene systems: From their structure-property relationship to DLP 3D printing. J. Mater. Chem. C 2018, 6, 11561–11568. [Google Scholar] [CrossRef]
- Wallin, T.J.; Pikul, J.H.; Bodkhe, S.; Peele, B.N.; Mac Murray, B.C.; Therriault, D.; McEnerney, B.W.; Dillon, R.P.; Giannelis, E.P.; Shepherd, R.F. Click chemistry stereolithography for soft robots that self-heal. J. Mater. Chem. B 2017, 5, 6249–6255. [Google Scholar] [CrossRef] [PubMed]
- Gragert, M.; Schunack, M.; Binder, W.H. Azide/alkyne-‘‘click’’-reactions of encapsulated reagents: Toward self-healing materials. Macromol. Rapid Commun. 2011, 32, 419–425. [Google Scholar] [CrossRef]
- Siddaramaiah, Q.L.; Kim, N.H.; Hui, D.; Lee, J.H. Effects of dual component microcapsules of resin and curing agent on the self-healing efficiency of epoxy. Compos. Part B 2013, 55, 79–85. [Google Scholar]
- Zhang, L.; Qiu, T.; Zhu, Z.; Guo, L.; Li, X. Self-healing polycaprolactone networks through thermo-induced reversible disulfide bond formation. Macromol. Rapid Commun. 2018, 39, 1800121. [Google Scholar] [CrossRef]
- Zhang, B.; Digby, Z.A.; Flum, J.A.; Chakma, P.; Saul, J.M.; Sparks, J.L.; Konkolewicz, D. Dynamic thiol−Michael chemistry for thermoresponsive rehealable and malleable networks. Macromolecules 2016, 49, 6871–6878. [Google Scholar] [CrossRef]
- Miao, W.; Zou, W.; Luo, Y.; Zheng, N.; Zhao, Q.; Xie, T. Structural tuning of polycaprolactone based thermadapt shape memory polymer. Polym. Chem. 2020, 11, 1369–1374. [Google Scholar] [CrossRef]
- Hillewaere, X.K.D.; Du Prez, F.E. Fifteen chemistries for autonomous external self-healing polymers and composites. Prog. Polym. Sci. 2015, 49–50, 121–153. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Scott, T.F.; Adzima, B.J.; Bowman, C.N. Covalent Adaptable Networks (CANs): A unique paradigm in cross-linked polymers. Macromolecules 2010, 43, 2643–2653. [Google Scholar] [CrossRef] [Green Version]
- Podgórski, M.; Fairbanks, B.D.; Kirkpatrick, B.E.; McBride, M.; Martinez, A.; Dobson, A.; Bongiardina, N.J.; Bowman, C.N. Toward stimuli-responsive dynamic thermosets through continuous development and improvements in covalent adaptable networks (CANs). Adv. Mater. 2020, 1906876. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Lei, Z.; Taynton, P.; Huang, S.; Zhang, W. Malleable and recyclable thermosets: The next generation of plastics. Matter 2019, 1, 1456–1493. [Google Scholar] [CrossRef] [Green Version]
- Scheutz, G.M.; Lessard, J.J.; Sims, M.B.; Sumerlin, B.S. Adaptable crosslinks in polymeric materials: Resolving the intersection of thermoplastics and thermosets. J. Am. Chem. Soc. 2019, 141, 16181–16196. [Google Scholar] [CrossRef] [PubMed]

























© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konuray, O.; Fernández-Francos, X.; De la Flor, S.; Ramis, X.; Serra, À. The Use of Click-Type Reactions in the Preparation of Thermosets. Polymers 2020, 12, 1084. https://doi.org/10.3390/polym12051084
Konuray O, Fernández-Francos X, De la Flor S, Ramis X, Serra À. The Use of Click-Type Reactions in the Preparation of Thermosets. Polymers. 2020; 12(5):1084. https://doi.org/10.3390/polym12051084
Chicago/Turabian StyleKonuray, Osman, Xavier Fernández-Francos, Silvia De la Flor, Xavier Ramis, and Àngels Serra. 2020. "The Use of Click-Type Reactions in the Preparation of Thermosets" Polymers 12, no. 5: 1084. https://doi.org/10.3390/polym12051084