In Situ Preparation of Amphibious ZnO Quantum Dots with Blue Fluorescence Based on Hyperbranched Polymers and their Application in Bio-Imaging
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis and Bio-Imaging Application
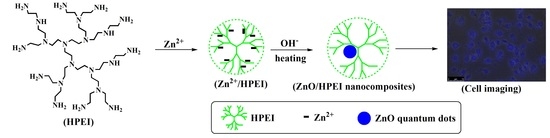
2.2.1. Synthesis of HPEI/Zn2+
2.2.2. Synthesis of Amphibious ZnO QDs with Blue Fluorescence Based on HPEI Hyperbranched Polymers
2.2.3. Bio-Imaging Application of ZnO/HPEI Nanocomposites with Blue Fluorescence
2.3. Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smith, A.M.; Nie, S.M. Semiconductor nanocrystals: Structure, properties, and band gap engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regulacio, M.D.; Han, M.Y. Composition-tunable alloyed semiconductor nanocrystals. Acc. Chem. Res. 2010, 43, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.N.; Yan, L.M.; Zhao, X.Y.; Chen, X.Y.; Li, A.H.; Zheng, D.; Zhou, X.; Dai, X.G.; Xu, F.J. Versatile types of organic/inorganic nanohybrids: From strategic design to biomedical applications. Chem. Rev. 2019, 119, 1666–1762. [Google Scholar] [CrossRef] [PubMed]
- Jalali, H.B.; Sadeghi, S.; Sahin, M.; Ozturk, H.; Ow-Yang, C.W.; Nizamoglu, S. Colloidal aluminum antimonide quantum dots. Chem. Mater. 2019, 31, 4743–4747. [Google Scholar] [CrossRef]
- Lu, H.P.; Carroll, G.M.; Neale, N.R.; Beard, M.C. Infrared quantum dots: Progress, challenges, and opportunities. ACS Nano 2019, 13, 939–953. [Google Scholar] [CrossRef]
- Li, M.X.; Chen, T.; Gooding, J.J.; Liu, J.Q. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Bhandari, S.; Hao, B.; Waters, K.; Lee, C.H.; Idrobo, J.C. Two-dimensional gold quantum dots with tunable bandgaps. ACS Nano 2019, 13, 4347–4353. [Google Scholar] [CrossRef]
- Gao, M.; Lesser, C.; Kirstein, S.; Möhwald, H.; Rogach, A.L.; Weller, H. Electroluminescence of different colors from polycation/CdTe nanocrystal self-assembled films. J. Appl. Phys. 2000, 87, 2297–2302. [Google Scholar] [CrossRef]
- Carey, G.H.; Abdelhady, A.L.; Ning, Z.J.; Thon, S.M.; Bakr, O.M.; Sargent, E.H. Colloidal quantum dot solar cells. Chem. Rev. 2015, 115, 12732–12763. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [Green Version]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisanic, T.R., II; Zhang, Y.; Wang, T.H. Quantum dots in diagnostics and detection: Principles and paradigms. Analyst 2014, 139, 2968–2981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegner, K.D.; Hildebrandt, N. Quantum dots: Bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem. Soc. Rev. 2015, 44, 4792–4834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.X.; Zeng, S.W.; Zhang, B.T.; Swihart, M.T.; Yong, K.T.; Prasad, P.N. New generation cadmium-free quantum dots for biophotonics and nanomedicine. Chem. Rev. 2016, 116, 12234–12327. [Google Scholar] [CrossRef]
- Chen, G.Y.; Roy, I.; Yang, C.H.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Adv. Drug Deliv. Rev. 2006, 58, 1456–1459. [Google Scholar] [CrossRef]
- Chen, O.; Chen, X.; Yang, Y.G.; Lynch, J.; Wu, H.M.; Zhuang, J.Q.; Cao, Y.C. Synthesis of metal–selenide nanocrystals using selenium dioxide as the selenium porecursor. Angew. Chem. Int. Ed. 2008, 47, 8638–8641. [Google Scholar] [CrossRef]
- Chen, O.; Zhao, J.; Chauhan, V.P.; Cui, J.; Wong, C.; Harris, D.K.; Wei, H.; Han, H.S.; Fukumura, D.; Jain, R.K.; et al. Compact high-quality CdSe–CdS core–shell nanocrystals with narrow emission linewidths and suppressed blinking. Nat. Mater. 2013, 12, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, N.; Battaglia, D.M.; Liu, Y.C.; Peng, X.G. Efficient, stable, small, and water-soluble doped ZnSe nanocrystal emitters as non-cadmium biomedical labels. Nano Lett. 2007, 7, 312–317. [Google Scholar] [CrossRef]
- Fu, Y.S.; Du, X.W.; Kulinich, S.A.; Qiu, J.S.; Qin, W.J.; Li, R.; Sun, J.; Liu, J. Stable aqueous dispersion of ZnO quantum dots with strong blue emission via simple solution route. J. Am. Chem. Soc. 2007, 129, 16029–16033. [Google Scholar] [CrossRef]
- Wang, Y.F.; He, L.; Yu, B.; Chen, Y.; Shen, Y.Q.; Cong, H.L. ZnO quantum dots modified by pH-activated charge-reversal polymer for tumor targeted drug delivery. Polymers 2018, 10, 1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Wolska-Pietkiewicz, M.; Badoni, S.; Grala, A.; Lewiński, J.; Paëpe, G.D. Disclosing interfaces of ZnO nanocrystals using dynamic nuclear polarization sol-gel versus organometallic approach. Angew. Chem. 2019, 131, 17323–17328. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asok, A.; Gandhi, M.N.; Kulkarni, A.R. Enhanced visible photoluminescence in ZnO quantum dots by promotion of oxygen vacancy formation. Nanoscale 2012, 4, 4943–4946. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.L.; Qian, X.F.; Gong, Q.; Du, W.M.; Ma, X.D.; Zhu, Z.K. Shape- and size-controlled synthesis of nanometre ZnO from a simple solution route at room temperature. Nanotechnology 2006, 17, 3632–3636. [Google Scholar] [CrossRef]
- Hu, Y.; Mei, T.; Guo, J.; White, T. Temperature-triggered self-assembly of ZnO: From nanocrystals to nanorods to tablets. Inorg. Chem. 2007, 46, 11031–11035. [Google Scholar] [CrossRef]
- Liu, D.P.; Li, G.D.; Su, Y.; Chen, J.S. Highly luminescent ZnO nanocrystals stabilized by ionic-liquid components. Angew. Chem. Int. Ed. 2006, 45, 7370–7373. [Google Scholar] [CrossRef]
- Xiong, H.M.; Liu, D.P.; Xia, Y.Y.; Chen, J. Polyether-grafted ZnO nanoparticles with tunable and stable photoluminescence at room temperature. Chem. Mater. 2005, 17, 3062–3064. [Google Scholar] [CrossRef]
- Xiong, H.M.; Wang, Z.D.; Liu, D.P.; Chen, J.S.; Wang, Y.G.; Xia, Y.Y. Bonding polyether onto ZnO nanoparticles: An effective method for preparing polymer nanocomposites with tunable luminescence and stable conductivity. Adv. Funct. Mater. 2005, 15, 1751–1756. [Google Scholar] [CrossRef]
- Saliba, S.; Serrano, C.V.; Keilitz, J.; Kahn, M.L.; Mingotaud, C.; Haag, R.; Marty, J.D. Hyperbranched polymers for the formation and stabilization of ZnO nanoparticles. Chem. Mater. 2010, 22, 6301–6309. [Google Scholar] [CrossRef]
- Xiong, H.M.; Xu, Y.; Ren, Q.G.; Xia, Y.Y. Stable aqueous ZnO@polymer core-shell nanoparticles with tunable photoluminescence and their application in cell imaging. J. Am. Chem. Soc. 2008, 130, 7522–7523. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.M.; Wang, Z.D.; Xia, Y.Y. Polymerization initiated by inherent free radicals on nanoparticle surfaces: A simple method of obtaining ultrastable ZnO@polymer core–shell nanoparticles with strong blue fluorescence. Adv. Mater. 2006, 18, 748–751. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xu, Y.D.; Ma, Y.Y.; Qiu, L.L.; Wang, Y.; Kong, J.L.; Xiong, H.M. Biodegradable ZnO@polymer core–shell nanocarriers: pH-triggered release of doxorubicin in vitro. Angew. Chem. 2013, 125, 4221–4225. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.H.; Chen, W.J.; Wu, Z.; Yuan, R.X.; Li, H.; Gao, J.M.; Shuai, X.T. MRI-visible polymeric vector bearing CD3 single chain antibody for gene delivery to T cells for immunosuppression. Biomaterials 2009, 30, 1962–1970. [Google Scholar] [CrossRef]
- Shi, Y.F.; Zhou, L.Z.; Wang, R.B.; Pang, Y.; Xiao, W.C.; Li, H.Q.; Su, Y.; Wang, X.L.; Zhu, B.S.; Zhu, X.Y.; et al. In situ preparation of magnetic nonviral gene vectors and magnetofection in vitro. Nanotechnology 2010, 21, 115103. [Google Scholar] [CrossRef]
- Shi, Y.F.; Du, J.M.; Zhou, L.Z.; Li, X.T.; Zhou, Y.H.; Li, L.L.; Zang, X.X.; Zhang, X.Y.; Pan, F.C.; Zhang, H.H.; et al. Size-controlled preparation of magnetic iron oxidenanocrystals within hyperbranched polymers and their magnetofection in vitro. J. Mater. Chem. 2012, 22, 355–360. [Google Scholar] [CrossRef]
- Crosby, G.A.; Demas, J.N. Measurement of photoluminescence quantum yields. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
- Green, M.; Harwood, H.; Barrowman, C.; Rahman, P.; Eggeman, A.; Festry, F.; Dobsonb, P.; Ng, T. A facile route to CdTe nanoparticles and their use in bio-labelling. J. Mater. Chem. 2007, 17, 1989–1994. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, G.; Yang, S.; Cao, R.; Zhou, P.; Peng, H.; Peng, R.; Zhang, X.; Yang, Y.; Li, Y.; Wang, M.; et al. In Situ Preparation of Amphibious ZnO Quantum Dots with Blue Fluorescence Based on Hyperbranched Polymers and their Application in Bio-Imaging. Polymers 2020, 12, 144. https://doi.org/10.3390/polym12010144
Lei G, Yang S, Cao R, Zhou P, Peng H, Peng R, Zhang X, Yang Y, Li Y, Wang M, et al. In Situ Preparation of Amphibious ZnO Quantum Dots with Blue Fluorescence Based on Hyperbranched Polymers and their Application in Bio-Imaging. Polymers. 2020; 12(1):144. https://doi.org/10.3390/polym12010144
Chicago/Turabian StyleLei, Gaiying, Shu Yang, Ranran Cao, Peng Zhou, Han Peng, Rui Peng, Xiaoming Zhang, Yujiao Yang, Yueyang Li, Mengyue Wang, and et al. 2020. "In Situ Preparation of Amphibious ZnO Quantum Dots with Blue Fluorescence Based on Hyperbranched Polymers and their Application in Bio-Imaging" Polymers 12, no. 1: 144. https://doi.org/10.3390/polym12010144