Polyamide-Laccase Nanofiber Membrane for Degradation of Endocrine-Disrupting Bisphenol A, 17α-ethinylestradiol, and Triclosan
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Electrospinning of the Nanofiber Matrix
2.3. Nanofiber Characterization
2.4. Enzyme Immobilization
2.4.1. Influence of Nanofiber Surface Density on the Immobilization Process
2.4.2. Influence of Laccase Solution Volume on the Immobilization Process
2.4.3. Effect of Buffer Concentration on the Immobilization Process
2.4.4. Effect of Time, pH and GA Concentration on the Immobilization Process
2.5. Enzyme Activity Assay
2.6. Storage Stability and Reusability of PA6-Laccase
2.7. Degradation of BPA, EE2, and TCS
3. Results and Discussion
3.1. Nanofiber Matrix Morphology
3.2. Optimal Volume of Laccase Solution
3.3. Optimal Buffer Concentration
3.4. Optimal Time for Adsorption and Crosslinking
3.5. Optimal pH and GA Concentration
3.6. Summary of the Optimal Immobilization Process
3.7. Storage Stability and Reuse
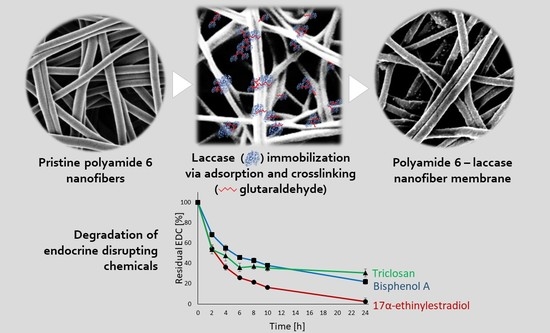
3.8. Degradation of BPA, EE2, and TCS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Gültekin, I.; Ince, N.H. Synthetic endocrine disruptors in the environment and water remediation by advanced oxidation processes. J. Environ. Manag. 2007, 85, 816–832. [Google Scholar] [CrossRef] [PubMed]
- Gmurek, M.; Olak-Kucharczyk, M.; Ledakowicz, S. Photochemical decomposition of endocrine disrupting compounds–A review. Chem. Eng. J. 2017, 310, 437–456. [Google Scholar] [CrossRef]
- EUR-Lex-32013L0039-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/2013/39/oj (accessed on 20 September 2019).
- Lin, T.; Wu, S.; Chen, W. Formation potentials of bromate and brominated disinfection by-products in bromide-containing water by ozonation. Environ. Sci. Pollut. Res. Int. 2014, 21, 13987–14003. [Google Scholar] [CrossRef] [PubMed]
- Matthis, A. Activated Carbon Options for Wastewater Treatment and Removal of Contaminants. Available online: https://www.watertechonline.com/activated-carbon-options-0517/ (accessed on 19 September 2019).
- Ge, J.C.; Choi, N.J. Performance of electrospun nanofibrous membranes for trapping of BTX aromatic hydrocarbons and heavy metal ions: Mechanisms, isotherms and kinetics. J. Clean. Prod. 2019, 217, 388–397. [Google Scholar] [CrossRef]
- Figoli, A.; Ursino, C.; Ramirez, D.O.S.; Carletto, R.A.; Tonetti, C.; Varesano, A.; Santo, M.P.D.; Cassano, A.; Vineis, C. Fabrication of electrospun keratin nanofiber membranes for air and water treatment. Polym. Eng. Sci. 2019, 59, 1472–1478. [Google Scholar] [CrossRef]
- Viswanath, B.; Rajesh, B.; Janardhan, A.; Kumar, A.P.; Narasimha, G. Fungal laccases and their applications in bioremediation. Enzym. Res. 2014, 2014, 163242. [Google Scholar] [CrossRef]
- Maryšková, M.; Ardao, I.; García-González, C.A.; Martinová, L.; Rotková, J.; Ševců, A. Polyamide 6/chitosan nanofibers as support for the immobilization of Trametes versicolor laccase for the elimination of endocrine disrupting chemicals. Enzym. Microb. Technol. 2016, 89, 31–38. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Price, W.E.; Leusch, F.D.L.; Roddick, F.; McAdam, E.J.; Magram, S.F.; Nghiem, L.D. Continuous biotransformation of bisphenol A and diclofenac by laccase in an enzymatic membrane reactor. Int. Biodeterior. Biodegrad. 2014, 95, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Cui, J.; Tang, R.; Li, F.; Zhang, B. Removal of 2,4,6-trichlorophenol by laccase immobilized on nano-copper incorporated electrospun fibrous membrane-high efficiency, stability and reusability. Chem. Eng. J. 2017, 326, 647–655. [Google Scholar] [CrossRef]
- Jahangiri, E.; Thomas, I.; Schulze, A.; Seiwert, B.; Cabana, H.; Schlosser, D. Characterisation of electron beam irradiation-immobilised laccase for application in wastewater treatment. Sci. Total Environ. 2018, 624, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Novel characteristics of horseradish peroxidase immobilized onto the polyvinyl alcohol-alginate beads and its methyl orange degradation potential. Int. J. Biol. Macromol. 2017, 105, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.P.; Rani, J.; Jaiswal, N.; Singh, S.; Awasthi, M.; Shasany, A.K.; Tiwari, S.; Dwivedi, U.N. Chitosan immobilized novel peroxidase from Azadirachta indica: Characterization and application. Int. J. Biol. Macromol. 2017, 104, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ling-Ling, H.; Yu-Zhi, D.; Yong-Juan, X.; Hua-Gang, N.; Cong, C.; Xiao-Lin, L.; Xiao-Jun, H. Hooking horseradish peroxidase by using the affinity Langmuir-Blodgett technique for an oriented immobilization. Appl. Surf. Sci. 2017, 403, 89–94. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Harbi, M.H.; Almulaiky, Y.Q.; Ibrahim, I.H.; El-Shishtawy, R.M. Immobilization of horseradish peroxidase on Fe3O4 magnetic nanoparticles. Electron. J. Biotechnol. 2017, 27, 84–90. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Akbulut, A.; Yakup Arica, M. Immobilization of tyrosinase on modified diatom biosilica: Enzymatic removal of phenolic compounds from aqueous solution. J. Hazard. Mater. 2013, 244, 528–536. [Google Scholar] [CrossRef]
- Marín-Zamora, M.E.; Rojas-Melgarejo, F.; García-Cánovas, F.; García-Ruiz, P.A. Production of o-diphenols by immobilized mushroom tyrosinase. J. Biotechnol. 2009, 139, 163–168. [Google Scholar] [CrossRef]
- Marín-Zamora, M.E.; Rojas-Melgarejo, F.; García-Cánovas, F.; García-Ruiz, P.A. Effects of the immobilization supports on the catalytic properties of immobilized mushroom tyrosinase: A comparative study using several substrates. J. Biotechnol. 2007, 131, 388–396. [Google Scholar] [CrossRef]
- Taboada-Puig, R.; Junghanns, C.; Demarche, P.; Moreira, M.T.; Feijoo, G.; Lema, J.M.; Agathos, S.N. Combined cross-linked enzyme aggregates from versatile peroxidase and glucose oxidase: Production, partial characterization and application for the elimination of endocrine disruptors. Bioresour. Technol. 2011, 102, 6593–6599. [Google Scholar] [CrossRef]
- Kochana, J.; Nowak, P.; Jarosz-Wilkołazka, A.; Bieroń, M. Tyrosinase/laccase bienzyme biosensor for amperometric determination of phenolic compounds. Microchem. J. 2008, 89, 171–174. [Google Scholar] [CrossRef]
- Ohtsu, T.; Shigenari, S.; Yoshimoto, M.; Umakoshi, H. Reactive bienzyme systems fabricated through immobilization of biotinylated glucose oxidase and peroxidase molecules onto neutralized avidin-conjugated liposomes. BioChem. Eng. J. 2017, 125, 81–87. [Google Scholar] [CrossRef]
- Ruan, C.; Li, Y. Detection of zeptomolar concentrations of alkaline phosphatase based on a tyrosinase and horse-radish peroxidase bienzyme biosensor. Talanta 2001, 54, 1095–1103. [Google Scholar] [CrossRef]
- McIlvaine, T.C. A Buffer Solution for Colorimetric Comparison. J. Biol. Chem. 1921, 49, 183–186. [Google Scholar]
- Hassani, T.; Ba, S.; Cabana, H. Formation of enzyme polymer engineered structure for laccase and cross-linked laccase aggregates stabilization. Bioresour. Technol. 2013, 128, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Ardao, I.; Magnin, D.; Agathos, S.N. Bioinspired production of magnetic laccase-biotitania particles for the removal of endocrine disrupting chemicals. Biotechnol. Bioeng. 2015, 112, 1986–1996. [Google Scholar] [CrossRef]
- Kim, J.; Jia, H.; Wang, P. Challenges in biocatalysis for enzyme-based biofuel cells. Biotechnol. Adv. 2006, 24, 296–308. [Google Scholar] [CrossRef]
- Fatarella, E.; Spinelli, D.; Ruzzante, M.; Pogni, R. Nylon 6 film and nanofiber carriers: Preparation and laccase immobilization performance. J. Mol. Catal. B Enzym. 2014, 102, 41–47. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Kamala-Kannan, S.; Cho, M.; Kim, J.S.; Hadibarata, T.; Salim, M.R.; Oh, B.-T. Laccase immobilization on cellulose nanofiber: The catalytic efficiency and recyclic application for simulated dye effluent treatment. J. Mol. Catal. B Enzym. 2014, 100, 111–120. [Google Scholar] [CrossRef]
- Xu, R.; Si, Y.; Wu, X.; Li, F.; Zhang, B. Triclosan removal by laccase immobilized on mesoporous nanofibers: Strong adsorption and efficient degradation. Chem. Eng. J. 2014, 255, 63–70. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Chae, J.-C.; Unnithan, A.R.; Palvannan, T.; Kim, H.Y.; Lee, K.-J.; Cho, M.; Kamala-Kannan, S.; Oh, B.-T. Laccase-poly (lactic-co-glycolic acid) (PLGA) nanofiber: Highly stable, reusable, and efficacious for the transformation of diclofenac. Enzym. Microb. Technol. 2012, 51, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Kumar, D.; Chauhan, G.S.; Kaushik, A. Preparation, characterization and trifluralin degradation of laccase-modified cellulose nanofibers. Mater. Sci. Energy Technol. 2018, 1, 29–37. [Google Scholar] [CrossRef]
- Wu, D.; Feng, Q.; Xu, T.; Wei, A.; Fong, H. Electrospun blend nanofiber membrane consisting of polyurethane, amidoxime polyarcylonitrile, and β-cyclodextrin as high-performance carrier/support for efficient and reusable immobilization of laccase. Chem. Eng. J. 2018, 331, 517–526. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Lin, L.; Xiao, G.; Tang, Z.; Zhu, X. Synthesis of novel laccase-biotitania biocatalysts for malachite green decolorization. J. Biosci. Bioeng. 2018, 126, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Estrada, C.; Rostro-Alanis, M.J.; Parra, A.L.; Belleville, M.-P.; Sanchez-Marcano, J.; Iqbal, H.M.N.; Parra-Saldívar, R. Potentialities of active membranes with immobilized laccase for Bisphenol A degradation. Int. J. Biol. Macromol. 2017, 108, 837–844. [Google Scholar] [CrossRef]
- Traunsteiner, C.; Sek, S.; Huber, V.; Valero-Vidal, C.; Kunze-Liebhäuser, J. Laccase immobilized on a mixed thiol monolayer on Au (111)–structure-dependent activity towards oxygen reduction. Electrochim. Acta 2016, 213, 761–770. [Google Scholar] [CrossRef]
- Reinikainen, T.; Teleman, O.; Teeri, T.T. Effects of pH and high ionic strength on the adsorption and activity of native and mutated cellobiohydrolase I from Trichoderma reesei. Proteins Struct. Funct. Bioinform. 2004, 22, 392–403. [Google Scholar] [CrossRef]
- Kim, R.E.; Hong, S.-G.; Ha, S.; Kim, J. Enzyme adsorption, precipitation and crosslinking of glucose oxidase and laccase on polyaniline nanofibers for highly stable enzymatic biofuel cells. Enzym. Microb. Technol. 2014, 66, 35–41. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, Q.; Li, F.; Zhang, B. Laccase immobilization on chitosan/poly (vinyl alcohol) composite nanofibrous membranes for 2,4-dichlorophenol removal. Chem. Eng. J. 2013, 222, 321–329. [Google Scholar] [CrossRef]
- Kadam, A.A.; Jang, J.; Jee, S.C.; Sung, J.-S.; Lee, D.S. Chitosan-functionalized supermagnetic halloysite nanotubes for covalent laccase immobilization. Carbohydr. Polym. 2018, 194, 208–216. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Yang, J.; Shinde, S.S.; Mistry, B.M.; Kim, D.-Y.; Sung, J.-S.; Kadam, A.A. Paper waste extracted α-cellulose fibers super-magnetized and chitosan-functionalized for covalent laccase immobilization. Bioresour. Technol. 2018, 261, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Drozd, R.; Rakoczy, R.; Wasak, A.; Junka, A.; Fijałkowski, K. The application of magnetically modified bacterial cellulose for immobilization of laccase. Int. J. Biol. Macromol. 2018, 108, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Skoronski, E.; Souza, D.H.; Ely, C.; Broilo, F.; Fernandes, M.; Fúrigo, A.; Ghislandi, M.G. Immobilization of laccase from Aspergillus oryzae on graphene nanosheets. Int. J. Biol. Macromol. 2017, 99, 121–127. [Google Scholar] [CrossRef] [PubMed]
- García-Morales, R.; García-García, A.; Orona-Navar, C.; Osma, J.F.; Nigam, K.D.P.; Ornelas-Soto, N. Biotransformation of emerging pollutants in groundwater by laccase from P. sanguineus CS43 immobilized onto titania nanoparticles. J. Environ. Chem. Eng. 2018, 6, 710–717. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2013, 4, 1583–1600. [Google Scholar] [CrossRef]
- Lassouane, F.; Aït-Amar, H.; Amrani, S.; Rodriguez-Couto, S. A promising laccase immobilization approach for Bisphenol A removal from aqueous solutions. Bioresour. Technol. 2018, 271, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Liu, Y.; Rouissi, T.; Pourcel, F.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Covalent immobilization of laccase on citric acid functionalized micro-biochars derived from different feedstock and removal of diclofenac. Chem. Eng. J. 2018, 351, 985–994. [Google Scholar] [CrossRef]
- López-Gallego, F.; Betancor, L.; Mateo, C.; Hidalgo, A.; Alonso-Morales, N.; Dellamora-Ortiz, G.; Guisán, J.M.; Fernández-Lafuente, R. Enzyme stabilization by glutaraldehyde crosslinking of adsorbed proteins on aminated supports. J. Biotechnol. 2005, 119, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Guibo, Y.; Qing, Z.; Yahong, Z.; Yin, Y.; Yumin, Y. The electrospun polyamide 6 nanofiber membranes used as high efficiency filter materials: Filtration potential, thermal treatment, and their continuous production. J. Appl. Polym. Sci. 2013, 128, 1061–1069. [Google Scholar] [CrossRef]
- Arica, M.Y.; Salih, B.; Celikbicak, O.; Bayramoglu, G. Immobilization of laccase on the fibrous polymer-grafted film and study of textile dye degradation by MALDI–ToF-MS. Chem. Eng. Res. Des. 2017, 128, 107–119. [Google Scholar] [CrossRef]
- Xu, R.; Tang, R.; Zhou, Q.; Li, F.; Zhang, B. Enhancement of catalytic activity of immobilized laccase for diclofenac biodegradation by carbon nanotubes. Chem. Eng. J. 2015, 262, 88–95. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Z.; Zeng, G.; Tang, L.; Pang, Y.; Li, Z.; Liu, C.; Lei, X.; Wu, M.; Ren, P.; et al. Immobilization of laccase on magnetic bimodal mesoporous carbon and the application in the removal of phenolic compounds. Bioresour. Technol. 2012, 115, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Demarche, P.; Agathos, S.N. Formulation and characterization of an immobilized laccase biocatalyst and its application to eliminate organic micropollutants in wastewater. New Biotechnol. 2013, 30, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Jankowska, K.; Wyszowska, M.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Pinelo, M.; Meyer, A.S.; Moszyński, D.; Jesionowski, T. Robust biodegradation of naproxen and diclofenac by laccase immobilized using electrospun nanofibers with enhanced stability and reusability. Mater. Sci. Eng. C 2019, 103, 109789. [Google Scholar] [CrossRef] [PubMed]
- Songulashvili, G.; Jimenéz-Tobón, G.A.; Jaspers, C.; Penninckx, M.J. Immobilized laccase of Cerrena unicolor for elimination of endocrine disruptor micropollutants. Fungal Biol. 2012, 116, 883–889. [Google Scholar] [CrossRef]
- Grieger, K.D.; Baun, A.; Owen, R. Redefining risk research priorities for nanomaterials. J. Nanopart. Res. 2010, 12, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Westerhoff, P.; Song, G.; Hristovski, K.; Kiser, M.A. Occurrence and removal of titanium at full scale wastewater treatment plants: Implications for TiO2 nanomaterials. J. Environ. Monit. 2011, 13, 1195–1203. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maryskova, M.; Rysova, M.; Novotny, V.; Sevcu, A. Polyamide-Laccase Nanofiber Membrane for Degradation of Endocrine-Disrupting Bisphenol A, 17α-ethinylestradiol, and Triclosan. Polymers 2019, 11, 1560. https://doi.org/10.3390/polym11101560
Maryskova M, Rysova M, Novotny V, Sevcu A. Polyamide-Laccase Nanofiber Membrane for Degradation of Endocrine-Disrupting Bisphenol A, 17α-ethinylestradiol, and Triclosan. Polymers. 2019; 11(10):1560. https://doi.org/10.3390/polym11101560
Chicago/Turabian StyleMaryskova, Milena, Miroslava Rysova, Vit Novotny, and Alena Sevcu. 2019. "Polyamide-Laccase Nanofiber Membrane for Degradation of Endocrine-Disrupting Bisphenol A, 17α-ethinylestradiol, and Triclosan" Polymers 11, no. 10: 1560. https://doi.org/10.3390/polym11101560