Molecular Dynamics Simulations on the Thermal Decomposition of Meta-Aramid Fibers
Abstract
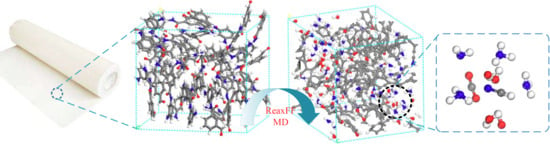
:1. Introduction
2. Modeling
3. Analysis of Simulation Results
3.1. Position of Initially Broken Bonds
3.2. Kinetic Calculation of Meta-Aramid Fiber Pyrolysis
3.3. Statistical Analysis of Major Thermal Decomposition Products
3.4. Generation Mechanism of NH3 and H2O
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wen, M.M.; Tian, M.Q.; Song, Y.; Liu, Y.; Lei, Z.P.; Song, J.C. Aging Law and Reliability Analysis of Nomex Paper Used for Dry-type Transformer Insulation. High Volt. Eng. 2014, 40, 3430–3437. [Google Scholar] [CrossRef]
- Schulten, H.R.; Plage, B.; Ohtani, H.; Tsuge, S. Studies on the thermal degradation of aromatic polyamides by pyrolysis-field ionization mass spectrometry and pyrolysis-gas chromatography. Angew. Makromol. Chem. 1987, 155, 1–20. [Google Scholar] [CrossRef]
- Wang, X.W.; Hu, Z.M.; Liu, Z.F. Study on the Pyrolytic Decomposition Process of Meta- and Para-Aramid Fibers by Py/GC-MS & TGA-DTA/MS. J. Instrum. Anal. 2008, 27, 349–354. [Google Scholar]
- Mettler, M.S.; Mushrif, S.H.; Paulsen, A.D.; Javadekar, A.D.; Vlachos, D.G.; Dauenhauer, P.J. Revealing pyrolysis chemistry for biofuels production: Conversion of cellulose to furans and small oxygenates. Energy Environ. Sci. 2012, 5, 5414–5424. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, V.; Dauenhauer, P.J.; Huber, G.W.; Auerbach, S.M. Ab initio dynamics of cellulose pyrolysis: Nascent decomposition pathways at 327 and 600 °C. J. Am. Chem. Soc. 2012, 134, 14958. [Google Scholar] [CrossRef] [PubMed]
- Warshel, A.; Weiss, R.M. Empirical valence bond calculations of enzyme catalysis. Ann. N. Y. Acad. Sci. 2010, 367, 370–382. [Google Scholar] [CrossRef]
- Nagy, T.; Yosa, R.J.; Meuwly, M. Multisurface Adiabatic Reactive Molecular Dynamics. J. Chem. Theory Comput. 2014, 10, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Brickel, S.; Meuwly, M. OH-Stretching Overtone Induced Dynamics in HSO3F from Reactive Molecular Dynamics Simulations. J. Phys. Chem. A 2017, 121, 5079–5087. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xiao, H.; Lackner, K.S.; Chen, X. Capture CO2 from Ambient Air Using Nanoconfined Ion Hydration. Angew. Chem. Int. Ed. Engl. 2016, 55, 4026–4029. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Bahri, K.; MüllerPlathe, F. Solid−Liquid and Solid−Solid Phase Diagrams of Self-Assembled Triblock Janus Nanoparticles from Solution. J. Phys. Chem. C 2018, 122, 9235–9244. [Google Scholar] [CrossRef]
- Hu, J.Y.; Liu, C.; Li, Q.B.; Shi, X.Y. Molecular simulation of thermal energy storage of mixed CO2/IRMOF-1 nanoparticle nanofluid. Int. J. Heat Mass Transf. 2018, 125, 1345–1348. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive Force Field for Hydrocarbons. J. Chem. Phys. 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wu, Y.J.; Chen, X.Y.; Wen, H.; Xiao, S. Theoretical Study on Decomposition Mechanism of Insulating Epoxy Resin Cured by Anhydride. Polymers 2017, 9, 341. [Google Scholar] [CrossRef]
- Strachan, A.; van Duin, A.C.T.; Chakraborty, D.; Dasgupta, S.; Goddard, W.A. Shock waves in high-energy materials: The initial chemical events in nitramine RDX. Phys. Rev. Lett. 2003, 91, 098301. [Google Scholar] [CrossRef] [PubMed]
- Nielson, K.D.; van Duin, A.C.T.; Oxgaard, J.; Deng, W.Q. Development of the ReaxFF reactive force field for describing transition metal catalyzed reactions, with application to the initial stages of the catalytic formation of carbon nanotubes. J. Phys. Chem. A 2005, 109, 493. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Truhlar, D.G.; Srinivasan, S.G.; van Duin, A.C.T.; Norman, P.; Schwartzentruber, T.E. Oxygen Interactions with Silica Surfaces: Coupled Cluster and Density Functional Investigation and the Development of a New ReaxFF Potential. J. Phys. Chem. C 2015, 117, 258–269. [Google Scholar] [CrossRef]
- Chenoweth, K.; van Duin, A.C.T.; Goddard, W. The ReaxFF Monte Carlo reactive dynamics method for predicting atomistic structures of disordered ceramics: Application to the Mo3VOx catalyst. Angew. Chem. Int. Ed. Engl. 2009, 48, 7630–7634. [Google Scholar] [CrossRef] [PubMed]
- Zandiatashbar, A.; Lee, G.H.; An, S.J.; Lee, S.; Mathew, N.; Terrones, M.; Hayashi, T.; Picu, C.R.; Hone, J.; Koratkar, N. Effect of defects on the intrinsic strength and stiffness of graphene. Nat. Commun. 2014, 5, 3186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.C.; Grossman, J.C. Atomistic understandings of reduced graphene oxide as an ultrathin-film nanoporous membrane for separations. Nat. Commun. 2015, 6, 8335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wen, Y.; Xue, X. Self-enhanced catalytic activities of functionalized graphene sheets in the combustion of nitromethane: Molecular dynamic simulations by molecular reactive force field. ACS Appl. Mater. Interfaces 2014, 6, 12235. [Google Scholar] [CrossRef] [PubMed]
- Beste, A. ReaxFF Study of the Oxidation of Lignin Model Compounds for the Most Common Linkages in Softwood in View of Carbon Fiber Production. J. Phys. Chem. A 2014, 118, 803. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Li, X.X.; Han, S.; Qiao, X.J.; Zhong, B.J.; Guo, L. Initial Reaction Mechanism of RP-3 High Temperature Oxidation Simulation With ReaxFF MD. Acta Phys. Chim. Sin. 2016, 32, 1424–1433. [Google Scholar] [CrossRef]
- Cheng, T.; Jaramillobotero, A.; Sun, H. Adaptive Accelerated ReaxFF Reactive Dynamics with Validation from Simulating Hydrogen Combustion. J. Am. Chem. Soc. 2014, 136, 9434. [Google Scholar] [CrossRef] [PubMed]
- Rom, N.; Zybin, S.V.; van Duin, A.C.T.; Goddard, W.A., III; Zeiri, Y.; Katz, G.; Kosloff, R. Density-dependent liquid nitromethane decomposition: Molecular dynamics simulations based on ReaxFF. J. Phys. Chem. A 2011, 115, 10181–10202. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Moghtaderi, B. Molecular Dynamics Simulation of the Low-Temperature Partial Oxidation of CH4. J. Phys. Chem. A 2009, 113, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, Q.M.; Zhang, Y.; Yang, R.; Gao, S.G. Simulation of Reactive Molecular Dynamics of Transformer Oil Pyrolysis at High Temperature and the Influence Mechanism of Acid in Oil. High Volt. Eng. 2017, 43, 247–255. [Google Scholar] [CrossRef]
- Yan, J.Y.; Wang, X.L.; Li, Q.M.; Zhou, Y.; Wang, Z.D.; Li, C.G. Molecular Dynamics Simulation on the Pyrolysis of Insulating Paper. Proc. CSEE 2015, 35, 5941–5949. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, T.; Shen, G.; Hou, Y.; Zou, L.; Zhang, L. Molecular dynamics simulation on generation mechanism of water molecules during pyrolysis of insulating paper. In Proceedings of the IEEE International Conference on High Voltage Engineering and Application, Chengdu, China, 19–22 September 2016; pp. 1–4. [Google Scholar]
- Castro-Marcano, F.; Russo, M.F., Jr.; van Duin, A.C.T.; Mathews, J.P. Pyrolysis of a large-scale molecular model for Illinois no. 6 coal using the ReaxFF reactive force field. J. Anal. Appl. Pyrol. 2014, 109, 79–89. [Google Scholar] [CrossRef]
- Jain, A.; Vijayan, K. Thermally induced structural changes in Nomex fibres. Bull. Mater. Sci. 2002, 25, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Varini, N.; English, N.J.; Trott, C.R. Molecular Dynamics Simulations of Clathrate Hydrates on Specialised Hardware Platforms. Energies 2012, 5, 3526–3533. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Li, X.; Li, Z.W.; Hao, J. Interfacial Hydrogen Bonds and Their Influence Mechanism on Increasing the Thermal Stability of Nano-SiO2-Modified Meta-Aramid Fibres. Polymers 2017, 9, 504. [Google Scholar] [CrossRef]
- Zha, W.; Song, H.T.; Dang, Z.M.; Shi, C.Y.; Bai, J.B. Mechanism analysis of improved corona-resistant characteristic in polyimide/TiO2 nanohybrid films. Appl. Phys. Lett. 2008, 93, 192911. [Google Scholar] [CrossRef]
- Dong, M.; Wang, H.; Shen, L.; Ye, Y.; Ye, C.; Wang, Y.; Zhang, J.; Jiang, Y. Dielectric property and electrical conduction mechanism of ZrO2–TiO2 composite thin films. J. Mater. Sci. Mater. Electron. 2012, 23, 174–179. [Google Scholar] [CrossRef]
- Yin, F.; Tang, C.; Li, X.; Wang, X.B. Effect of Moisture on Mechanical Properties and Thermal Stability of Meta-Aramid Fiber used in Insulating Paper. Polymers 2017, 9, 537. [Google Scholar] [CrossRef]
- Zha, J.W.; Meng, X.; Wang, D.R.; Dang, Z.M.; Li, R.K.Y. Dielectric properties of poly(vinylidene fluoride) nanocomposites filled with surface coated BaTiO3 by SnO2 nanodots. Appl. Phys. Lett. 2014, 104, 072906. [Google Scholar] [CrossRef]
- Bourbigot, S.; Flambard, X. Heat resistance and flammability of high performance fibres: A review. Fire Mater. 2010, 26, 155–168. [Google Scholar] [CrossRef]
- Zhang, S.F. Correlation between the Interface and Structure Characteristics of Meta-Aramid Fiber and the Properties for Sheetmaking. Ph.D. Thesis, Shaanxi University of Science & Technology, Xi’an, China, 2009. [Google Scholar]
- Ding, J.; Zhang, L.; Zhang, Y.; Han, K.L. A reactive molecular dynamics study of n-heptane pyrolysis at high temperature. J. Phys. Chem. A 2013, 117, 3266–3278. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.L.; Wu, G.Y.; Chen, C.L. Investigation of Thermal Cracking Process of n-Decane by Molecular Dynamics Simulation. Acta Pet. Sin. 2001, 17, 77–82. [Google Scholar]
- Mayo, S.L.; Olafson, B.D.; Goddard, W.A. DREIDING: A generic force field for molecular simulations. J. Phys. Chem. 1990, 94, 8897–8909. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Wang, C.; Wu, J.Y. Molecular Dynamics Study of the Effect of H2O on the Thermal Decomposition of α Phase CL-20. Proc. CSEE 2013, 29, 1145–1153. [Google Scholar] [CrossRef]
- Chen, B.; Diao, Z.J.; Lu, H.Y. Using the ReaxFF reactive force field for molecular dynamics simulations of the spontaneous combustion of lignite with the Hatcher lignite model. Fuel 2014, 116, 7–13. [Google Scholar] [CrossRef]
- Eslami, H.; Heydari, N. Hydrogen bonding in water nanoconfined between graphene surfaces: A molecular dynamics simulation study. J. Nanopart. Res. 2013, 16, 1–10. [Google Scholar] [CrossRef]
- Afandak, A.; Eslami, H. Ion-Pairing and Electrical Conductivity in the Ionic Liquid 1-n-Butyl-3-methylimidazolium Methylsulfate [Bmim][MeSO4]: Molecular Dynamics Simulation Study. J. Phys. Chem. B 2017, 121, 7699–7708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Research on Micro-Mechanism of Pyrolysis Process of Oil-Paper Insulation in Power Transformer. Master’s Thesis, North China Electric Power University, Beijing, China, 2016. [Google Scholar]










| Chemical bond | Car–N | Caromatic ring-Ccarbonyl | C=O | Other |
|---|---|---|---|---|
| Broken times | 15 | 1 | 10 | 4 |
| Proportion (%) | 50 | 3 | 33 | 14 |
| T(K) | 2000 | 2300 | 2500 | 2700 | 3000 |
| 1/T(K−1) | 0.00050 | 0.00043 | 0.00040 | 0.00037 | 0.00033 |
| lnk | 24.67 | 25.34 | 25.86 | 26.45 | 27.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, F.; Tang, C.; Wang, Q.; Liu, X.; Tang, Y. Molecular Dynamics Simulations on the Thermal Decomposition of Meta-Aramid Fibers. Polymers 2018, 10, 691. https://doi.org/10.3390/polym10070691
Yin F, Tang C, Wang Q, Liu X, Tang Y. Molecular Dynamics Simulations on the Thermal Decomposition of Meta-Aramid Fibers. Polymers. 2018; 10(7):691. https://doi.org/10.3390/polym10070691
Chicago/Turabian StyleYin, Fei, Chao Tang, Qian Wang, Xiong Liu, and Yujing Tang. 2018. "Molecular Dynamics Simulations on the Thermal Decomposition of Meta-Aramid Fibers" Polymers 10, no. 7: 691. https://doi.org/10.3390/polym10070691