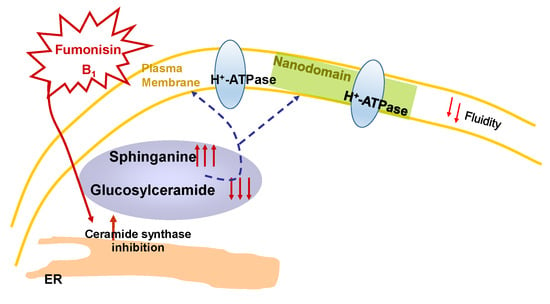
Sphingolipid Effects on the Plasma Membrane Produced by Addition of Fumonisin B1 to Maize Embryos
Abstract
:1. Introduction
2. Results
2.1. FB1 Addition to the Maize Embryos Inhibits the PM H+-ATPase Activity
2.2. Sphingolipid Species Vary in the PM from Maize Embryos Exposed to FB1
2.3. Changes in Sphingolipids as a Result of FB1 Addition to the Maize Embryos are Associated to an Increase in the Permeability and a Decrease in the Fluidity of the PM
2.4. FB1 Addition Does Not Modify the Profile of the PM Main Fatty Acids
2.5. LCBs but Not Ceramide Inhibit H+-ATPase Activity When Directly Added to the PM
2.6. Ceramide Releases the FB1 Inhibition on the PM H+-ATPase Activity, When Both Compounds are Added Together to the Maize Embryos
3. Discussion
3.1. Effects of FB1 on the Lipid Bilayer of the PM from Maize Embryos
3.2. Effect of FB1 on the PM H+-ATPase Activity from Maize Embryos
3.3. Relevance of the Effects of FB1 in the Plant–Pathogen Interaction
4. Materials and Methods
4.1. Imbibition of Maize Embryos
4.2. Measurement of Electrolyte Leakage
4.3. Isolation of PM Vesicles
4.4. Determination of ATP Hydrolysis
4.5. Measurement of PM Fluidity
4.6. Determination of FB1
4.7. Measurement of LCBs from PM
4.8. Measurement of Fatty Acids from PM
4.9. Detection of Complex Sphingolipids by TLC
4.10. Immunoblotting Assays
4.11. Protein Determination
4.12. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bidway, A.P.; Zhang, L.; Bachmann, R.C.; Takemoto, J.Y. Mechanism of action of Pseudomonas syringae phytotoxin. Stimulation of red beet plasma membrane ATPase activity. Plant Physiol. 1987, 83, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Batoko, H.; d’Exaerde, A.-K.; Kinet, J.-M.; Bouharmont, J.; Gage, A.R.; Maraite, H.; Boutry, M. Modulation of plant plasma membrane H+-ATPase by phytotoxic lipodepsipeptides produced by the plant pathogen Pseudomonas fuscovaginae. Biochim. Biophys. Acta 1998, 1372, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Goudet, C.; Véry, A.A.; Milat, M.L.; Ildefonse, M.; Thibaud, J.B.; Sentenac, H.; Blein, J.P. Magnesium ions promote assembly of channel-like structures from beticolin 0, a non-peptide fungal toxin purified from Cercospora beticola. Plant J. 1998, 14, 359–364. [Google Scholar] [CrossRef]
- Shier, W.T. Sphingosine analogs: An emerging new class of toxins that includes the fumonisins. J. Toxicol. 1992, 11, 241–257. [Google Scholar] [CrossRef]
- Doehlert, D.C.; Knutson, C.A.; Vesonder, R.F. Phytotoxic effects of fumonisin B1 on maize seedling growth. Mycopathologia 1994, 127, 117–121. [Google Scholar] [CrossRef]
- Gelderblom, W.C.; Galendo, D.; Abel, S.; Swanevelder, S.; Marasas, W.F.; Wild, C.P. Cancer initiation by fumonisin B(1) in rat liver—Role of cell proliferation. Cancer Lett. 2001, 169, 127–137. [Google Scholar] [CrossRef]
- Stockmann-Juvala, H.; Savolainen, K. A review of the toxic effects and mechanisms of action of fumonisin B1. Hum. Exp. Toxicol. 2008, 27, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Kriek, N.P.; Marasas, W.F.; Thiel, P.G. Hepato- and cardiotoxicity of Fusarium verticillioides (F. moniliforme) isolates from Southern African maize. Food Cosmet. Toxicol. 1981, 19, 447–456. [Google Scholar] [CrossRef]
- Gelderblom, W.C.; Jaskiewicz, K.; Marasas, W.F.; Thiel, P.G.; Horak, R.M.; Vleggaar, R.; Kriek, N.P. Fumonisins—Novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1988, 54, 1806–1811. [Google Scholar] [CrossRef] [Green Version]
- Nelson, P.E.; Desjardins, A.E.; Plattner, R.D. Fumonisins, mycotoxins produced by Fusarium species: Biology, chemistry, and significance. Annu. Rev. Phytopathol. 1993, 31, 233–252. [Google Scholar] [CrossRef]
- Marasas, W.F. Discovery and occurrence of the fumonisins: A historical perspective. Environ. Health Perspect. 2001, 109, 239–243. [Google Scholar] [PubMed]
- Yogendrarajah, P.; Jacxsens, L.; Lachat, C.; Walpita, C.N.; Kolsteren, P.; De Saeger, S.; De Meulenaer, B. Public health risk associated with the co-occurrence of mycotoxins in spices consumed in Sri Lanka. Food Chem. Toxicol. 2014, 74, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.S.; Tang, L.; Sun, G.; Wang, S.; Hu, X.; Wang, J.-S. Mycotoxin exposure is associated with increased risk of esophageal squamous cell carcinoma in Huaian area, China. BMC Cancer 2019, 19, 1218–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wielogorska, E.; Mooney, M.; Eskola, M.; Ezekiel, C.N.; Stranska, M.; Krska, R.; Elliott, C. Occurrence and Human-Health Impacts of Mycotoxins in Somalia. J. Agric. Food Chem. 2019, 67, 2052–2060. [Google Scholar] [CrossRef]
- Abbas, H.K.; Paul, R.N.; Boyette, C.D.; Duke, S.O.; Vesonder, R.F. Physiological and ultrastructural effects of fumonisin on jimsonweed leaves. Can. J. Bot. 1992, 70, 1824–1833. [Google Scholar] [CrossRef]
- Gutiérrez-Nájera, N.; Muñoz-Clares, R.A.; Palacios-Bahena, S.; Ramírez, J.; Sánchez-Nieto, S.; Plasencia, J.; Gavilanes-Ruíz, M. Fumonisin B1, a sphingoid toxin, is a potent inhibitor of the plasma membrane H+-ATPase. Planta 2005, 221, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.-J.; Smith, M.J.; Eppley, R.M.; Page, S.W.; Sphon, J.A. Effects of fumonisin B1 on oxygen transport in membranes. Biochim. Biophys. Res. Commun. 1996, 225, 250–255. [Google Scholar] [CrossRef]
- Yin, J.J.; Smith, M.J.; Eppley, R.M.; Page, S.W.; Sphon, J.A. Effects of fumonisin B1 on lipid peroxidation in membranes. Biochim. Biophys. Acta 1998, 1371, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Sondergaard, T.E.; Schulz, A.; Palmgren, M.G. Energization of transport processes in plants. Roles of the plasma membrane H+-ATPase. Plant Physiol. 2004, 136, 2475–2482. [Google Scholar] [CrossRef] [Green Version]
- Haruta, M.; Gray, W.M.; Sussman, M.R. Regulation of the plasma membrane proton pump (H+-ATPase) by phosphorylation. Curr. Opin. Plant Biol. 2015, 28, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Falhoff, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Bielawski, J.; Mu, J.; Dong, H.; Teng, C.; Zhang, J.; Yang, X.; Tomishige, N.; Hanada, K.; Hannun, Y.A.; et al. Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis. Cell Res. 2007, 17, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-García, M.; Guevara-García, A.; González-Solís, A.; Cruz-García, F.; Vázquez-Santana, S.; Markham, J.E.; Lozano-Rosas, M.G.; Dietrich, C.R.; Ramos-Vega, M.; Cahoon, E.B.; et al. MPK6, sphinganine and the LCB2a gene from serine palmitoyltransferase are required in the signaling pathway that mediates cell death induced by long chain bases in Arabidopsis. New Phytol. 2011, 191, 943–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, E.; Norred, W.P.; Bacon, C.W.; Riley, R.T.; Merrill, A.H., Jr. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 1991, 266, 14486–14490. [Google Scholar] [PubMed]
- Abado-Becognee, K.; Mobio, T.A.; Ennamany, R.; Fleurat-Lessard, F.; Shier, W.T.; Badria, F.; Creppy, E.E. Cytotoxicity of fumonisin B1: Implication of lipid peroxidation and inhibition of protein and DNA synthesis. Arch. Toxicol. 1998, 72, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Salazar, L.; El Hafidi, M.; Gutiérrez-Nájera, N.; Noyola-Martínez, L.; González-Solís, A.; Gavilanes-Ruíz, M. Fatty acid profiles from the plasma membrane and detergent resistant membranes of two plant species. Phytochemistry 2015, 129, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.I.; McDonough, V.M.; Nickels, J.T., Jr.; Ko, J.; Fischl, A.S.; Vales, T.R.; Merrill, A.H., Jr.; Carman, G.M. Regulation of lipid biosynthesis in Saccharomyces cerevisiae by fumonisin B1. J. Biol. Chem. 1995, 270, 13171–13178. [Google Scholar] [CrossRef] [Green Version]
- Asai, T.; Stone, J.M.; Heard, J.E.; Kovtun, Y.; Yorgey, P.; Sheen, J.; Ausubel, F.M. Fumonisin B1-induced cell death in Arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signaling pathways. Plant Cell 2000, 12, 1823–1835. [Google Scholar]
- Stone, J.M.; Heard, J.E.; Asai, T.; Ausubel, F.M. Simulation of fungal-mediated cell death by fumonisin B1 and selection of fumonisin B1-resistant (fbr) Arabidopsis mutants. Plant Cell 2000, 12, 1811–1822. [Google Scholar]
- Desai, K.; Sullards, M.C.; Allegood, J.; Wang, E.; Schmelz, E.M.; Hartl, M.; Humpf, H.V.; Liotta, D.C.; Peng, Q.; Merrill, A.H., Jr. Fumonisins and fumonisin analogs as inhibitors of ceramide synthase and inducers of apoptosis. Biochim. Biophys. Acta 2002, 1585, 188–192. [Google Scholar] [CrossRef]
- Massey, J.B. Interaction of ceramides with phosphatidylcholine, sphingomyelin and sphingomyelin/cholesterol bilayers. Biochim. Biophys. Acta 2011, 510, 167–184. [Google Scholar] [CrossRef] [Green Version]
- Contreras, F.-X.; Sot, J.; Alonso, A.; Goñi, F.M. Sphingosine increases the permeability of model and cell membranes. Biophys. J. 2006, 90, 4085–4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siskind, L.J.; Fluss, S.; Bui, M.; Colombini, M. Sphingosine forms channels in membranes that differ greatly from those formed by ceramide. J. Bioenerg. Biomem. 2005, 4, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, H.K.; Tanaka, T.; Duke, O.S.; Porter, J.K.; Wray, E.M.; Hodges, L.; Sessions, A.E.; Wang, E.; Merrill, H.A., Jr.; Riley, R.T.; et al. Fumonisin and AAL-toxin-induced disruption of sphingolipid metabolism with accumulation of free sphingoid bases: Involvement in plant disease. Plant Physiol. 1994, 106, 1085–1093. [Google Scholar] [CrossRef] [Green Version]
- London, M.; London, E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): Implications for lipid raft structure and function. J. Biol. Chem. 2004, 279, 9997–10004. [Google Scholar]
- Gaigg, B.; Timischl, B.; Corbino, L.; Schneiter, R. Synthesis of sphingolipids with very long chain fatty acids but not ergosterol is required for routing of newly synthesized plasma membrane ATPase to the cell surface of yeast. J. Biol. Chem. 2005, 280, 22515–22522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, D.T.; Burden, R.S.; James, C.S.; Seco, T.; Sierra, B. Influence of sterols on plasma membrane proton-pumping ATPase activity and membrane fluidity in oat shoots. Plant Physiol. Biochem. 1994, 32, 769–773. [Google Scholar]
- Smith, W.; Merrill, A.H. Jr. Sphingolipid metabolism and signaling minireview series. J. Biol. Chem. 2002, 277, 25841–25842. [Google Scholar] [CrossRef] [Green Version]
- Kanczewska, J.; Marco, S.; Vandermeeren, C.; Maudoux, O.; Rigaud, J.L.; Boutry, M. Activation of the plant plasma membrane H+-ATPase by phosphorylation and binding of 14-3-3 proteins converts a dimer into a hexamer. Proc. Natl. Acad. Sci. USA 2005, 102, 11675–11680. [Google Scholar] [CrossRef] [Green Version]
- Idkowiak-, J.; Grilley, M.M.; Takemoto, J.Y. Sphingolipid C4 hydroxylation influences properties of yeast detergent-insoluble glycolipid-enriched membranes. FEBS Lett. 2004, 569, 272–276. [Google Scholar] [CrossRef] [Green Version]
- Mongrand, S.; Morel, J.; Laroche, J.; Claverol, S.; Carde, J.P.; Hartmann, M.A.; Bonneu, M.; Simon-Plas, F.; Lessire, R.; Bessoule, J.J. Lipid rafts in higher plant cells: Purification and characterization of Triton X-100-insoluble microdomains from tobacco plasma membrane. J. Biol. Chem. 2004, 279, 36277–36286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borner, G.H.H.; Sherrier, D.J.; Weimar, T.; Michaelson, L.V.; Hawkins, N.D.; MacAskill, A.; Napier, J.A.; Beale, M.H.; Lilley, K.S.; Dupree, P. Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol. 2005, 137, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, J.; Claverol, S.; Mongrand, S.; Furt, F.; Fromentin, J.; Bessoule, J.J.; Blein, J.P.; Simon-Plas, F. Proteomics of plant detergent-resistant membranes. Mol. Cell Proteom. 2006, 5, 1396–1411. [Google Scholar] [CrossRef] [Green Version]
- Simons, K.; Vaz, W.L. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Salazar, L.; El Hafidi, M.; Enríquez-Arredondo, C.; Vázquez-Vázquez, C.; González de la Vara, L.E.; Gavilanes-Ruíz, M. Isolation of detergent-resistant membranes from plant photosynthetic and non-photosynthetic tissues. Anal. Biochem. 2011, 417, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J. How sphingolipids bind and shape proteins: Molecular basis of lipid-protein interactions in lipid shells, rafts and related biomembrane domains. Cell. Mol. Life Sci. 2003, 60, 1027–1032. [Google Scholar] [CrossRef]
- Gong, X.; Chang, A. A mutant plasma membrane ATPase, Pma1-10, is defective in stability at the yeast cell surface. Proc. Natl. Acad. Sci. USA 2001, 98, 9104–9109. [Google Scholar] [CrossRef] [Green Version]
- Cowan, D.S.C.; Cooke, D.T.; Clarkson, D.T.; Hall, J.L. Lipid and sterol composition of plasma membranes isolated from stele and cortex of maize roots. J. Exp. Bot. 1993, 44, 991–994. [Google Scholar] [CrossRef]
- Burgos, P.A.; Donaire, J.P. Phospholipids, free sterols, fluidity and ATPase activity of plasma membrane-enriched vesicles from sunflower and jojoba roots. Plant Physiol. Biochem. 1996, 34, 315–324. [Google Scholar]
- Holthuis, J.C.; Pomorski, T.; Raggers, R.J.; Sprong, H.; Van Meer, G. The organizing potential of sphingolipids in intracellular membrane transport. Physiol. Rev. 2001, 81, 1689–1723. [Google Scholar] [CrossRef]
- Xu, X.; Bittman, R.; Duportail, G.; Heissler, D.; Vilcheze, C.; London, E. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J. Biol. Chem. 2001, 276, 33540–33546. [Google Scholar] [PubMed] [Green Version]
- Gronnier, J.; Gerbeau-Pissot, P.; Germain, V.; Mongrand, S.; Simon-Plas, F. Divide and rule: Plant plasma membrane organization. Trends Plant Sci. 2018, 23, 899–917. [Google Scholar] [CrossRef] [PubMed]
- Bohn, M.; Heinz, E.; Lüthje, S. Lipid composition and fluidity of plasma membranes isolated from corn (Zea mays L.) roots. Arch. Biochem. Biophys. 2001, 387, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Vera-Estrella, R.; Barkla, B.J.; Higgins, V.J.; Blumwald, E. Plant defense response to fungal pathogens (activation of host-plasma membrane H+-ATPase by elicitor-induced enzyme dephosphorylation). Plant Physiol. 1994, 104, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Speth, C.; Jaspert, N.; Marcon, C.; Oecking, C. Regulation of the plant plasma membrane H+-ATPase by its C-terminal domain: What do we know for sure? Eur. J. Cell Biol. 2010, 89, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.K.; Carr, K.; McAinsh, M.R.; Powell, B.; Hetherington, A.M. Drought-induced guard cell signal transduction involves sphingosine-1-phosphate. Nature 2001, 410, 596–599. [Google Scholar] [CrossRef]
- Coursol, S.; Stunff, H.; Lynch, D.V.; Gilroy, S.; Assmann, S.M.; Spiegel, S. Arabidopsis sphingosine kinase and the effects of phytosphingosine-1-phosphate on stomatal aperture. Plant Physiol. 2005, 137, 724–737. [Google Scholar] [CrossRef] [Green Version]
- Hannun, Y.; Luberto, C.; Argraves, K.M. Enzymes of sphingolipid metabolism: From modular to integrative signaling. Biochemistry 2001, 40, 4893–4903. [Google Scholar] [CrossRef]
- Markham, J.E.; Li, J.; Cahoon, E.B.; Jaworski, J.G. Separation and identification of major plant sphingolipid classes from leaves. J. Biol. Chem. 2006, 281, 22684–22694. [Google Scholar] [CrossRef] [Green Version]
- Lynch, D.; Chen, M.; Cahoon, B.E. Lipid signaling in Arabidopsis: No sphingosine? No problem! Trends Plant Sci. 2009, 14, 463–466. [Google Scholar] [CrossRef]
- Riley, R.T.; Merrill, A.H. Ceramide synthase inhibition by fumonisins: A perfect storm of perturbed sphingolipid metabolism, signaling and disease. J. Lipid Res. 2019, 60, 1183–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaller, A.; Oecking, C. Modulation of plasma membrane H+-ATPase activity differentially activates wound and pathogen defense responses in tomato plants. Plant Cell 1999, 11, 263–272. [Google Scholar] [PubMed] [Green Version]
- Elmore, J.M.; Coaker, G. The role of the plasma membrane H+-ATPase in plant–microbe interactions. Mol. Plant 2011, 4, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, S.L.; Mary, V.S.; Otaiza, S.N.; Wunderlin, D.A.; Rubinstein, H.R.; Theumer, M.G. Toxin distribution and sphingoid base imbalances in Fusarium verticillioides-infected and fumonisin B1-watered maize seedlings. Phytochemistry 2016, 125, 54–64. [Google Scholar] [CrossRef]
- Sánchez-Nieto, S.; García-Rubio, O.; Pacheco-Moisés, F.; Carballo, A.; Rodríguez-Sotres, R.; Gavilanes-Ruíz, M. Purification of plasma membranes from dry maize embryos. Physiol. Plant 1997, 101, 157–164. [Google Scholar] [CrossRef]
- González-Romo, P.; Sánchez-Nieto, S.; Gavilanes-Ruíz, M. A modified colorimetric method for the determination of orthophosphate in the presence of high ATP concentrations. Anal. Biochem. 1992, 200, 235–238. [Google Scholar] [CrossRef]
- Sánchez-Nieto, S.; de Gómez-Puyou, M.T.; Rodríguez-Sotres, R.; Carballo, A.; Gavilanes-Ruíz, M. Comparison of plasma membrane H+-ATPase activity in vesicles obtained from dry and hydrated maize embryos. Biochim. Biophys. Acta 1998, 1414, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Sydenham, E.W.; Shephard, G.S.; Thiel, P.G. Liquid chromatographic determination of fumonisins B1, B2, B3 in foods and feeds. J. AOAC Int. 1992, 75, 313–318. [Google Scholar]
- Castegnaro, M.; Garren, L.; Galendo, D.; Gelderblom, W.C.A.; Chelule, P.; Dutton, M.F.; Wild, C.P. Analytical method for the determination of sphinganine and sphingosine in serum as a potential biomarker for fumonisin exposure. J. Chromatogr. B 1998, 720, 15–24. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, G.; Uematsu, S.; Akahori, Y.; Hirabayashi, Y. Induction of apoptotic DNA fragmentation and cell death by natural ceramide. FEBS Lett. 1995, 358, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Taki, T.; Kasama, T.; Hannada, S.; Ishikawa, D. A simple and quantitative purification of glycosphingolipids and phospholipids by thin layer chromatography blotting. Anal. Biochem. 1994, 223, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Deckert, J.V.E. Sphingolipid extraction and analysis by thin layer chromatography. Method. Enzymol. 2000, 312, 64–79. [Google Scholar]
- Enríquez-Arredondo, C.; Sánchez-Nieto, S.; Rendón-Huerta, E.; González-Halphen, D.; Gavilanes-Ruíz, M.; Díaz-Pontones, D. The plasma membrane H+-ATPase of maize embryos localizes in regions that are critical during the onset of germination. Plant Sci. 2005, 169, 11–19. [Google Scholar] [CrossRef]
- Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Nájera, N.A.; Saucedo-García, M.; Noyola-Martínez, L.; Vázquez-Vázquez, C.; Palacios-Bahena, S.; Carmona-Salazar, L.; Plasencia, J.; El-Hafidi, M.; Gavilanes-Ruiz, M. Sphingolipid Effects on the Plasma Membrane Produced by Addition of Fumonisin B1 to Maize Embryos. Plants 2020, 9, 150. https://doi.org/10.3390/plants9020150
Gutiérrez-Nájera NA, Saucedo-García M, Noyola-Martínez L, Vázquez-Vázquez C, Palacios-Bahena S, Carmona-Salazar L, Plasencia J, El-Hafidi M, Gavilanes-Ruiz M. Sphingolipid Effects on the Plasma Membrane Produced by Addition of Fumonisin B1 to Maize Embryos. Plants. 2020; 9(2):150. https://doi.org/10.3390/plants9020150
Chicago/Turabian StyleGutiérrez-Nájera, Nora A., Mariana Saucedo-García, Liliana Noyola-Martínez, Christian Vázquez-Vázquez, Silvia Palacios-Bahena, Laura Carmona-Salazar, Javier Plasencia, Mohammed El-Hafidi, and Marina Gavilanes-Ruiz. 2020. "Sphingolipid Effects on the Plasma Membrane Produced by Addition of Fumonisin B1 to Maize Embryos" Plants 9, no. 2: 150. https://doi.org/10.3390/plants9020150