Blue Light Enhances Cadmium Tolerance of the Aquatic Macrophyte Potamogeton crispus
Abstract
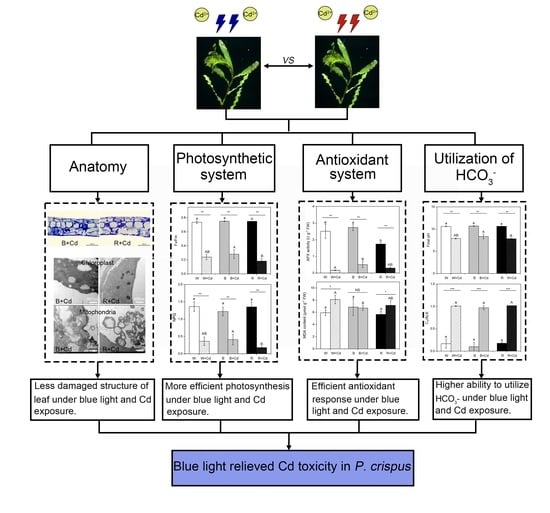
:1. Introduction
2. Results
2.1. Leaf Morphology and Anatomic Structure
2.2. Ultrastructure of Chloroplasts and Mitochondria
2.3. Pigment Content, Chlorophyll Fluorescence, and Photosynthetic Rate
2.4. HCO3− Uptake
2.5. MDA Content, In Situ O2.− Accumulation, and Antioxidant Enzyme Activity
3. Discussion
3.1. The Effect of Light Qualities on Leaf Anatomy and the Ultrastructure of Chloroplasts and Mitochondria of P. crispus under Cd Stress
3.2. The Effect of Light Qualities on Pigment, Photochemistry, and HCO3− Uptake of P. crispus under Cd Stress
3.3. The Effect of Light Qualities on the Antioxidant System of P. crispus under Cd Stress
4. Materials and Methods
4.1. Plant Material and Pre-Treatment Culture Conditions
4.2. Spectral and Cd Treatments
4.3. Observation of Leaf Anatomy and the Ultrastructure of Chloroplast and Mitochondria
4.4. Measurements of Pigment Content and Chlorophyll Fluorescence
4.5. Measurement of Photosynthetic Rate
4.6. pH-Drift Experiments
4.7. Histochemical Detection of Superoxide (O2.−) and Measurements of Malondialdehyde (MDA) Content and Antioxidant Enzyme Activity
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.L.; Jeppesen, E.; Liu, X.H.; Qin, B.Q.; Shi, K.; Zhou, Y.Q.; Thomaz, S.M.; Deng, J.M. Global loss of aquatic vegetation in lakes. Earth-Sci. Rev. 2017, 173, 259–265. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Simultaneous removal of organics and heavy metals from industrial wastewater: A review. Chemosphere 2021, 262, 128379. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, M.T.; Aguiar, F.C.; Asaeda, T.; Bakker, E.S.; Chambers, P.A.; Clayton, J.S.; Elger, A.; Ferreira, T.M.; Gross, E.M.; Gunn, I.D.M.; et al. Plants in aquatic ecosystems: Current trends and future directions. Hydrobiologia 2018, 812, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Srivastava, S.; Tripathi, R.D.; Govindarajan, R.; Kuriakose, S.V.; Prasad, M.N.V. Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol. Biochem. 2006, 44, 25–37. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, response to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Haider, F.U.; Cai, L.Q.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.Z.; Ma, W.J.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotox. Environ. Safe. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Sanità di Toppi, L.; Vurro, E.; Rossi, L.; Marabottini, R.; Musetti, R.; Careri, M.; Maffini, M.; Mucchino, C.; Corradini, C.; Badiani, M. Different compensatory mechanisms in two metal-accumulating aquatic macrophytes exposed to acute cadmium stress in outdoor artificial lakes. Chemosphere 2007, 68, 769–780. [Google Scholar] [CrossRef]
- Huang, W.M.; Shao, H.; Zhou, S.N.; Zhou, Q.; Li, W.; Xing, W. Modulation of cadmium-induced phytotoxicity in Cabomba caroliniana by urea involves photosynthetic metabolism and antioxidant status. Ecotox. Environ. Safe. 2017, 144, 88–96. [Google Scholar] [CrossRef]
- Schutzendubel, A.; Polle, A. Plant responses to abiotic stress: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef]
- Asada, K. Ascorbate peroxidase—A hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant. 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Dong, X.H.; Yang, X.D.; Liu, E.F.; Lin, Q.; Cheng, L.J.; Liu, L.; Jeppesen, E. Aquatic macrophyte fluctuations since the 1900s in the third largest Chinese freshwater lake (Lake Taihu): Evidences, drivers and management implications. Catena 2022, 213, 106153. [Google Scholar] [CrossRef]
- Wang, L.Y.; Han, S.J.; Wang, S.W.; Li, W.; Huang, W.M. Morphological, photosynthetic, and CAM physiological responses of the submerged macrophyte Ottelia alismoides to light quality. Environ. Exp. Bot. 2022, 202, 105002. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Ahmadi, T.; Shabani, L.; Sabzalian, M.R. Improvement in drought tolerance of lemon balm, Melissa officinalis L. under the pre-treatment of LED lighting. Plant Physiol. Biochem. 2019, 139, 548–557. [Google Scholar] [CrossRef]
- Kirk, J.T.O. (Ed.) Light and Photosynthesis in Aquatic Ecosystems; Cambridge University: Cambridge, UK, 2011. [Google Scholar]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of photosynthesis after the ‘red light syndrome’ in cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Tehrani, P.F.; Majd, A.; Mahmoodzadeh, H.; Satari, T.N. Effect of red and blue light–emitting diodes on germination, morphological and anatomical features of Brassica napus. Adv. Stud. Biol. 2016, 8, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Pettai, H.; Oja, V.; Freiberg, A.; Laisk, A. Photosynthetic activity of far-red light in green plants. BBA–Bioenerg. 2005, 1708, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 17, 4657–4670. [Google Scholar] [CrossRef] [Green Version]
- Li, J.F.; Yi, C.Y.; Zhang, C.R.; Pan, F.; Xie, C.; Zhou, W.Z.; Zhou, C.F. Effects of light quality on leaf growth and photosynthetic fluorescence of Brasenia schreberi seedlings. Heliyon 2021, 7, e06082. [Google Scholar] [CrossRef]
- Huang, W.M.; Han, S.J.; Zhou, Q.; Li, W.; Xing, W. Assessing interactions between environmental factors and aquatic toxicity: Influences of dissolved CO2 and light on Cd toxicity in the aquatic macrophyte Potamogeton crispus. Aquat. Toxicol. 2019, 212, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.X.; Lv, J.L.; Zhang, H.M.; Hu, C.Y.; Qin, Y.P.; Dong, H.; Zhang, T.; Dong, X.X.; Du, N.S.; Piao, F.Z. Red and blue light function antagonistically to regulate cadmium tolerance by modulating the photosynthesis, antioxidant defense system and Cd uptake in cucumber (Cucumis sativus L.). J. Hazard. Mater. 2022, 429, 128412. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.S.; Min, H.L.; Cai, S.J.; Fu, Y.Y.; Sha, S.; Xie, K.B.; Du, K.H. Subcellular distribution and toxicity of cadmium in Potamogeton crispus L. Chemosphere 2012, 89, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Sivaci, A.; Elmas, E.; Gümüş, F. Removal of cadmium by Myriophyllum heterophyllum Michx. and Potamogeton crispus L. and its effect on pigments and total phenolic compounds. Arch. Environ. Contam. Toxicol. 2008, 54, 612–618. [Google Scholar] [CrossRef]
- Artetxe, U.; García-Plazaola, J.I.; Hernández, A.; Becerril, J.M. Low light grown duckweed plants are more protected against the toxicity induced by Zn and Cd. Plant Physiol. Bioch. 2002, 40, 859–863. [Google Scholar] [CrossRef]
- Huang, W.M.; Jin, Q.; Yin, L.Y.; Li, W. Responses of CO2-concentrating mechanisms and photosynthetic characteristics in aquatic plant Ottelia alismoides following cadmium stress under low CO2. Ecotox. Environ. Safe. 2020, 202, 110955. [Google Scholar] [CrossRef]
- Zeshan, A.; Abdullah, M.; Adil, M.F.; Wei, D.M.; Noman, M.; Ahmed, T.; Sehar, S.; Ouyang, Y.N.; Shamsi, I.H. Improvement of morpho-physiological, ultrastructural and nutritional profiles in wheat seedlings through astaxanthin nanoparticles alleviating the cadmium toxicity. J. Hazard. Mater. 2022, 424, 126511. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, R.; Ju, Q.; Li, W.; Lam-Son, P.T.; Xu, J. The R2R3-MYB transcription factor MYB49 regulates cadmium accumulation. Plant Physiol. 2019, 180, 529–542. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. (Eds.) Biochemistry & Molecular Biology of Plants; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Neves, N.R.; Oliva, M.A.; Centeno, D.C.; Costa, A.C.; Ribas, R.F.; Pereira, E.G. Photosynthesis and oxidative stress in the resting plant species Eugenia uniflora L. exposed to simulated acid rain and iron ore dust deposition: Potential use in environmental risk assessment. Sci. Total Environ. 2009, 407, 3740–3745. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [Green Version]
- Dobrikova, A.G.; Apostolova, E.L.; Hanć, A.; Yotsova, E.; Borisova, P.; Sperdouli, I.; Adamakis, I.D.S.; Moustakase, M. Cadmium toxicity in Salvia sclarea L.: An integrative response of element uptake, oxidative stress markers, leaf structure and photosynthesis. Ecotox. Environ. Safe. 2021, 209, 111851. [Google Scholar] [CrossRef]
- Marchello, A.E.; Oliveira, N.L.; Lombardi, A.T.; Polpo, A. An investigation onto Cd toxicity to freshwater microalga Chlorella sorokiniana in mixotrophy and photoautotrophy: A Bayesian approach. Chemosphere 2018, 211, 794–803. [Google Scholar] [CrossRef]
- Andresen, E.; Kappel, S.; Stärk, H.J.; Riegger, U.; Borovec, J.; Mattusch, J.; Heinz, A.; Schmelzer, C.E.; Matoušková, Š.; Dickinson, B.; et al. Cadmium toxicity investigated at the physiological and biophysical levels under environmentally relevant conditions using the aquatic model plant Ceratophyllum demersum. New Phytol. 2016, 210, 1244–1258. [Google Scholar] [CrossRef]
- Wang, S.; Wufuer, R.; Duo, J.; Li, W.; Pan, X. Cadmium caused different toxicity to photosystem I and photosystem II of freshwater unicellular algae Chlorella pyrenoidosa (Chlorophyta). Toxics 2022, 10, 352. [Google Scholar] [CrossRef]
- Iversen, L.L.; Winkel, A.; Baastrup-Spohr, L.; Hinke, A.B.; Alahuhta, J.; Baattrup-Pedersen, A.; Birk, S.; Brodersen, P.; Chambers, P.A.; Ecke, F.; et al. Catchment properties and the photosynthetic trait composition of freshwater plant communities. Science 2019, 366, 878–881. [Google Scholar] [CrossRef]
- Maberly, S.C.; Gontero, B. Ecological imperatives for aquatic CO2-concentrating mechanisms. J. Exp. Bot. 2017, 68, 3797–3814. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.M.; Han, S.J.; Jiang, H.S.; Gu, S.P.; Li, W.; Gontero, B.; Maberly, S.C. External α-carbonic anhydrase and solute carrier 4 are required for bicarbonate uptake in a freshwater angiosperm. J. Exp. Bot. 2020, 71, 6004–6014. [Google Scholar] [CrossRef]
- Wang, S.N.; Li, P.P.; Liao, Z.Y.; Wang, W.W.; Chen, T.; Yin, L.Y.; Jiang, H.S.; Li, W. Adaptation of inorganic carbon utilization strategies in submerged and floating leaves of heteroblastic plant Ottelia cordata. Environ. Exp. Bot. 2022, 196, 104818. [Google Scholar] [CrossRef]
- Maberly, S.C. Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae. J. Phycol. 1990, 26, 439–449. [Google Scholar] [CrossRef]
- Maberly, S.C.; Spence, D.H.N. Photosynthetic inorganic carbon use by freshwater plants. J. Ecol. 1983, 71, 705–724. [Google Scholar] [CrossRef]
- Maberly, S.C.; Gontero, B. Trade-offs and synergies in the structural and functional characteristics of leaves photosynthesizing in aquatic environments. In The Leaf: A Platform for Performing Photosynthesis; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; pp. 307–343. [Google Scholar]
- Tóth, T.; Zsiros, O.; Kis, M.; Garab, G.; Kovacs, L. Cadmium exerts its toxic effects on photosynthesis via a cascade mechanism in the cyanobacterium, Synechocystis PCC 6803. Plant Cell Environ. 2012, 35, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Jie, Y.; Xu, D.; Ai, K.; Bao, E.; Zou, Z. Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol. 2018, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, L.; Chen, X.; Wu, X.; Xiang, X.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; Foyer, C.H.; et al. SlHY5 integrates temperature, light, and hormone signaling to balance plant growth and cold tolerance. Plant Physiol. 2018, 179, 749–760. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.T.; Li, Z.L.; He, J.J.; Wang, X.Y.; Liu, Q.L.; Huang, J.; Xie, Y.D.; He, Z.Q. Effects of red to far-red light ratio on growth and photosynthetic characteristics of tomato seedlings under calcium nitrate stress. Photosynthetica 2021, 59, 625–632. [Google Scholar] [CrossRef]
- Chen, L.B.; Huang, J.; Liu, Q.L.; Li, Z.L.; Chen, X.; Han, J.X.; Gan, Y.R.; He, Y.X.; Jiang, C.X.; Tang, Y.X.; et al. Low R/FR ratio affects Pakchos growth and nitrate content under excess nitrate stress. Horticulturae 2022, 8, 186. [Google Scholar] [CrossRef]
- Gaion, L.A.; Lorevice, P.G.; Monteiro, C.C.; Gavassi, M.A.; D’Amico-Damião, V.; Gratão, P.L.; Gasparino, E.C.; Carvalho, R.F. The role of phytochromes in cadmium stress responses in tomato. Bragantia 2017, 77, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Tolerance and hyper-accumulation of cadmium by a wild, unpalatable herb Coronopus didymus (L.) Sm. (Brassicaceae). Ecotox. Environ. Safe. 2017, 135, 209–215. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, Y.H.; Liu, T.; He, Y.H. Leaf defense system of Robinia pseudoacacia L. seedlings exposed to 3 years of elevated atmospheric CO2 and Cd-contaminated soils. Sci. Total Environ. 2017, 605–606, 48–57. [Google Scholar] [CrossRef]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hurst, R.D.; Hancock, J.T. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 2002, 53, 1237–1247. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Głowacka, K.; Źróbek-Sokolnik, A.; Okorski, A.; Najdzion, J. The effect of cadmium on the activity of stress-related enzymes and the ultrastructure of Pea Roots. Plants 2019, 8, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muradoglu, F.; Gundogdu, M.; Ercisli, S.; Encu, T.; Balta, F.; Jaafar, H.Z.; Zia-Ul-Haq, M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015, 48, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dat, J.; Vandenabeele, S.; Vranov, E.; van Montagu, M.; Inze, D.; van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, M.; Ayub, F.; Shah, A.A.; Naz, G.; Shah, A.N.; Malik, A.; Sardar, R.; Telesiński, A.; Kalaji, H.M.; Dessoky, E.S.; et al. Synergistic effect of zinc oxide nanoparticles and Moringa oleifera leaf extract alleviates cadmium toxicity in Linum usitatissimum: Antioxidants and physiochemical studies. Front. Plant Sci. 2022, 13, 900347. [Google Scholar] [CrossRef] [PubMed]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [Green Version]
- Shaw, P. Effects of mercury and cadmium on the activities of antioxidative enzymes in the seedlings of Phaseolus aureus. Biol. Plant 1995, 37, 587–596. [Google Scholar] [CrossRef]
- Ali, M.B.; Chun, H.S.; Kim, B.K.; Lee, C.B. Cadmium-induced changes in antioxidant enzyme activities in rice (Oryza sativa L. cv. Dongjin). J. Plant Biol. 2002, 45, 134–140. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Han, S.J.; Maberly, S.C.; Gontero, B.; Xing, Z.F.; Jiang, H.S.; Huang, W.M. Structural basis for C4 photosynthesis without kranz anatomy in leaves of the submerged freshwater plant Ottelia alismoides. Ann. Bot. 2020, 125, 869–879. [Google Scholar] [CrossRef]
- Brain, R.A.; Solomon, K.R. A protocol for conducting 7-day daily renewal tests with Lemna gibba. Nat. Protoc. 2007, 2, 979–987. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Maberly, S.C. Diel, episodic and seasonal changes in pH and concentrations of inorganic carbon in a productive lake. Freshwater Biol. 1996, 35, 579–598. [Google Scholar] [CrossRef]
- Liu, N.; Jin, Z.Y.; Wang, S.S.; Gong, B.; Wen, D.; Wang, X.F.; Wei, M.; Shi, Q.H. Sodic alkaline stress mitigation with exogenous melatonin involves reactive oxygen metabolism and ion homeostasis in tomato. Sci. Hortic. 2015, 181, 18–25. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]






| Item | Source | ||
|---|---|---|---|
| Cd | Light Quality | Cd × Light Quality | |
| Chl a | 3597.64 *** | 6.19 ** | 12.80 *** |
| Chl b | 1838.20 *** | 2.77 NS | 7.01 ** |
| Total Chl | 3124.70 *** | 5.10 * | 11.27 ** |
| Chl a/b | 98.38 *** | 0.93 NS | 1.00 NS |
| Car | 1612.05 *** | 2.34 NS | 3.33 NS |
| Chl/Car | 129.58 *** | 2.34 NS | 4.76 * |
| Fv/Fm | 1036.18 *** | 3.23 NS | 3.24 NS |
| YⅡ | 365.54 *** | 2.87 NS | 0.97 NS |
| qP | 104.64 *** | 12.74 ** | 5.20 * |
| NPQ | 282.63 *** | 0.86 NS | 3.19 NS |
| ETRmax | 225.84 *** | 0.96 NS | 0.77 NS |
| α | 934.50 *** | 0.73 NS | 5.10 * |
| Final pH | 797.54 *** | 4.27 * | 2.23 NS |
| CO2 | 179.04 *** | 1.01 NS | 1.01 NS |
| HCO3− | 1197.90 *** | 1.17 NS | 1.18 NS |
| CT/ALK | 1720.08 *** | 0.28 NS | 0.84 NS |
| MDA | 10.63 ** | 1.42 NS | 6.08 * |
| SOD | 1188.38 *** | 14.49 ** | 14.49 ** |
| CAT | 63.14 *** | 8.84 ** | 6.66 * |
| GR | 0.08 NS | 6.36 * | 7.30 ** |
| APX | 419.65 *** | 12.49 ** | 8.15 ** |
| Treatments | O2 Evolution Rate (mg O2 h−1 g−1 FW) |
|---|---|
| W + Cd | No Detect |
| B + Cd | 1.83 ± 0.79 |
| R + Cd | No Detect |
| Treatments | Final pH | ALK (equiv L−1) | CT (mmol L−1) | CO2 (μmol L−1) | HCO3− (mmol L−1) | CO32− (mmol L−1) | CT/ALK |
|---|---|---|---|---|---|---|---|
| W | 10.64 ± 0.11 (a,α) | 1.00 ± 0.10 (a,α) | 0.17 ± 0.11 (a,α) | 0.002 ± 0.001 (a,α) | 0.04 ± 0.02 (a,α) | 0.14 ± 0.08 (a,α) | 0.17 ± 0.10 (a,α) |
| W + Cd | 7.90 ± 0.12 (AB,β) | 1.11 ± 0.12 (A,α) | 1.13 ± 0.13 (A,β) | 25.22 ± 9.97 (AB,β) | 1.10 ± 0.12 (A,β) | 0.01 ± 0.00 (A,β) | 1.01 ± 0.01 (A,β) |
| B | 10.73 ± 0.09 (a,α) | 1.04 ± 0.03 (a,α) | 0.11 ± 0.05 (a,α) | 0.001 ± 0.000 (a,α) | 0.04 ± 0.02 (a,α) | 0.16 ± 0.05 (a,α) | 0.10 ± 0.10 (a,α) |
| B + Cd | 8.36 ± 0.53 (A,β) | 1.06 ± 0.06 (A,α) | 1.03 ± 0.10 (A,β) | 12.12 ± 10.42 (A,β) | 0.99 ± 0.12 (A,β) | 0.01 ± 0.00 (A,β) | 0.97 ± 0.05 (A,β) |
| R | 10.66 ± 0.02 (a,α) | 1.04 ± 0.01 (a,α) | 0.18 ± 0.02 (a,α) | 0.001 ± 0.000 (a,α) | 0.04 ± 0.00 (a,α) | 0.15 ± 0.01 (a,α) | 0.18 ± 0.02 (a,α) |
| R + Cd | 7.84 ± 0.06 (B,β) | 1.06 ± 0.02 (A,α) | 1.08 ± 0.02 (A,β) | 26.97 ± 3.70 (B,β) | 1.04 ± 0.02 (A,β) | 0.01 ± 0.00 (A,β) | 1.02 ± 0.00 (A,β) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, L.; Zhang, M.; Li, W.; Xie, Z.; Huang, W. Blue Light Enhances Cadmium Tolerance of the Aquatic Macrophyte Potamogeton crispus. Plants 2023, 12, 2667. https://doi.org/10.3390/plants12142667
Wang S, Wang L, Zhang M, Li W, Xie Z, Huang W. Blue Light Enhances Cadmium Tolerance of the Aquatic Macrophyte Potamogeton crispus. Plants. 2023; 12(14):2667. https://doi.org/10.3390/plants12142667
Chicago/Turabian StyleWang, Shanwei, Liyuan Wang, Miao Zhang, Wei Li, Zuoming Xie, and Wenmin Huang. 2023. "Blue Light Enhances Cadmium Tolerance of the Aquatic Macrophyte Potamogeton crispus" Plants 12, no. 14: 2667. https://doi.org/10.3390/plants12142667