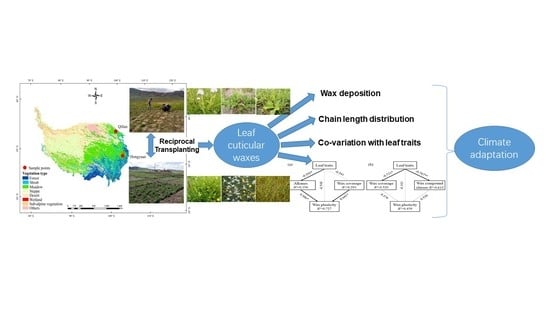
Phenotypic Plasticity and Local Adaptation of Leaf Cuticular Waxes Favor Perennial Alpine Herbs under Climate Change
Abstract
:1. Introduction
2. Results
2.1. Responses of Leaf Wax Coverage and Wax Compositions
2.2. The Response Coefficient (RC) and Phenotypic Plasticity Index (PI) of Leaf Wax Traits
2.3. Covariation of Phenotypic Plasticity Indices among Traits
3. Discussion
3.1. Cuticular Waxes of Plants Were Changed in Order to Adapt to New Environments
3.2. The Phenotypic Plasticity of Waxes Traits Improved the Plant Adaptations
4. Materials and Methods
4.1. Experimental Design
4.2. Soil Chemical Analysis
4.3. Plant Traits Measurements
4.4. Leaf Cuticular Waxes Extraction
4.5. GC and GC/MS Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Espeland, E.K.; Kettenring, K.M. Strategic plant choices can alleviate climate change impacts: A review. J. Environ. Manag. 2018, 222, 316–324. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.E. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef]
- Bradshaw, A.D. Unravelling phenotypic plasticity—Why should we bother? New Phytol. 2006, 170, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Jetter, R.; Kunst, L.; Samuels, A.L. Composition of plant cuticular waxes. In Biology of the Plant Cuticle; Riederer, M., Muller, C., Eds.; Annual Plant Reviews (Series); The University of British Columbia: Vancouver, BC, Canada, 2006; Volume 23, pp. 145–181. [Google Scholar]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Guo, N.; He, Y.; Gao, J. Cuticular waxes in alpine meadow plants: Climate effect inferred from latitude gradient in Qinghai-Tibetan Plateau. Ecol. Evol. 2015, 5, 3954–3968. [Google Scholar] [CrossRef]
- Bush, R.T.; McInerney, F.A. Leaf wax n-alkane distributions in and across modern plants: Implications for paleoecology and chemotaxonomy. Geochim. Cosmochim. Acta 2013, 117, 161–179. [Google Scholar] [CrossRef]
- Duan, Y.; He, J. Distribution and isotopic composition of n-alkanes from grass, reed and tree leaves along a latitudinal gradient in China. Geochem. J. 2011, 45, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Dodd, R.S.; Poveda, M.M. Environmental gradients and population divergence contribute to variation in cuticular wax composition in Juniperus communis. Biochem. Syst. Ecol. 2003, 31, 1257–1270. [Google Scholar] [CrossRef]
- Carnahan, A.M.; Spalinger, D.E.; Collins, W.B. n-Alkane and long-chain alcohol recovery in moose (Alces alces), a browsing herbivore. Can. J. Zool. 2018, 96, 559–565. [Google Scholar] [CrossRef]
- Braccini, C.L.; Vega, A.S.; Araoz, M.V.C.; Teal, P.E.; Cerrillo, T.; Zavala, J.A.; Fernandez, P.C. Both volatiles and cuticular plant compounds determine oviposition of the Willow Sawfly Nematus oligospilus on leaves of Salix spp. (Salicaceae). J. Chem. Ecol. 2015, 41, 985–996. [Google Scholar] [CrossRef]
- Eigenbrode, S.D. The effects of plant epicuticular waxy blooms on attachment and effectiveness of predatory insects. Arthropod. Struct. Deve. 2004, 33, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Suseela, V.; Tharayil, N. Decoupling the direct and indirect effects of climate on plant litter decomposition: Accounting for stress-induced modifications in plant chemistry. Glob. Chang. Biol. 2018, 24, 1428–1451. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, T.; Griffiths, D.W. The effects of stress on plant cuticular waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef] [PubMed]
- Henn, J.J.; Buzzard, V.; Enquist, B.J.; Halbritter, A.H.; Klanderuds, K.; Maitner, B.S.; Michaletz, S.T.; Potschs, C.; Seltzer, L.; Telford, R.J.; et al. Intraspecific trait variation and phenotypic plasticity mediate alpine plant species response to climate change. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.; Moore, T.E.; Mollmann, H.K.; Carlson, J.E.; Mocko, K.; Martinez-Cabrera, H.; Adams, C.; Silander, J.A., Jr.; Jones, C.S.; Schlichting, C.D.; et al. Functional traits in parallel evolutionary radiations and trait-environment associations in the cape floristic region of south Africa. Am. Nat. 2015, 185, 525–537. [Google Scholar] [CrossRef]
- Liancourt, P.; Boldgiv, B.; Song, D.S.; Spence, L.A.; Helliker, B.R.; Petraitis, P.S.; Casper, B.B. Leaf-trait plasticity and species vulnerability to climate change in a Mongolian steppe. Glob. Chang. Biol. 2015, 21, 3489–3498. [Google Scholar] [CrossRef]
- Richards, T.J.; Ortiz-Barrientos, D.; McGuigan, K. Natural selection drives leaf divergence in experimental populations of Senecio lautus under natural conditions. Ecol. Evol. 2019, 9, 6959–6967. [Google Scholar] [CrossRef] [Green Version]
- Poorter, L. Leaf traits show different relationships with shade tolerance in moist versus dry tropical forests. New Phytol. 2009, 181, 890–900. [Google Scholar] [CrossRef]
- Schreiber, S.G.; Hacke, U.G.; Hamann, A. Variation of xylem vessel diameters across a climate gradient: Insight from a reciprocal transplant experiment with a widespread boreal tree. Funct. Ecol. 2015, 29, 1392–1401. [Google Scholar] [CrossRef]
- Ran, F.; Zhang, X.; Zhang, Y.; Korpelainen, H.; Li, C. Altitudinal variation in growth, photosynthetic capacity and water use efficiency of Abies faxoniana Rehd. et Wils. seedlings as revealed by reciprocal transplantations. Trees-Struct. Funct. 2013, 27, 1405–1416. [Google Scholar] [CrossRef]
- Cui, H.; Topper, J.P.; Yang, Y.; Vandvik, V.; Wang, G. Plastic populationeffects and conservative leaf traits in a reciprocal transplant experiment simulating climate warming in the Himalayas. Front. Plant Sci. 2018, 9, 1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallfors, M.; Lehvavirta, S.; Aandahl, T.; Lehtimaki, I.-M.; Nilsson, L.O.; Ruotsalainen, A.; Schulman, L.E.; Hyvarinen, M.T. Translocation of an arctic seashore plant reveals signs of maladaptation to altered climatic conditions. PeerJ 2020, 8, e10357. [Google Scholar] [CrossRef] [PubMed]
- Kesselring, H.; Hamann, E.; Armbruster, G.F.J.; Stocklin, J.; Scheepens, J.F. Local adaptation is stronger between than within regions in alpine populations of Anthyllis vulneraria. Evol. Ecol. 2019, 33, 737–750. [Google Scholar] [CrossRef]
- Guo, Y.J.; Zhao, X.; Li, Y.; Li, Z.; Xiao, Q.L.; Wang, Y.M.; Zhang, X.F.; Ni, Y. Environment-driven adaptations of leaf cuticular waxes are inheritable for Medicago ruthenica. Front. Plant Sci. 2021, 12, 620245. [Google Scholar] [CrossRef]
- Bernard, A.; Joubes, J. Arabidopsis cuticular waxes: Advances in synthesis, export and regulation. Prog. Lipid Res. 2013, 52, 110–129. [Google Scholar] [CrossRef] [PubMed]
- Bourdenx, B.; Bernard, A.; Domergue, F.; Pascal, S.; Leger, A.; Roby, D.; Pervent, M.; Vile, D.; Haslam, R.P.; Napier, J.A.; et al. Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant Physiol. 2011, 156, 29–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heredia-Guerrero, J.A.; Guzman-Puyol, S.; Benitez, J.J.; Athanassiou, A.; Heredia, A.; Dominguez, E. Plant cuticle under global change: Biophysical implications. Glob. Chang. Biol. 2018, 24, 2749–2751. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Nga, N.; Hykkerud, A.L.; Haggman, H.; Martinussen, I.; Jaakola, L.; Karppinen, K. Developmental and environmental regulation of cuticular wax biosynthesis in fleshy fruits. Front. Plant Sci. 2019, 10, 431. [Google Scholar] [CrossRef]
- Verrall, B.; Pickering, C.M. Alpine vegetation in the context of climate change: A global review of past research and future directions. Sci. Total Environ. 2020, 748, 141344. [Google Scholar] [CrossRef]
- Midolo, G.; Kuss, P.; Wellstein, C. Land use and water availability drive community-level plant functional diversity of grasslands along a temperature gradient in the Swiss Alps. Sci. Total Environ. 2021, 764, 142888. [Google Scholar] [CrossRef]
- Halbritter, A.H.; Fior, S.; Keller, I.; Billeter, R.; Edwards, P.J.; Holderegger, R.; Karrenberg, S.; Pluess, A.R.; Widmer, A.; Alexander, J.M. Trait differentiation and adaptation of plants along elevation gradients. J. Evolution. Biol. 2018, 31, 784–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midolo, G.; Wellstein, C. Plant performance and survival across transplant experiments depend upon temperature and precipitation change along elevation. J. Ecol. 2020, 108, 2107–2120. [Google Scholar] [CrossRef]
- Kim, E.; Donohue, K. Local adaptation and plasticity of Erysimum capitatum to altitude: Its implications for responses to climate change. J. Ecol. 2013, 101, 796–805. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Duan, Y.-W.; Hao, G.; Ge, X.-J.; Sun, H. Evolutionary history and underlying adaptation of alpine plants on the Qinghai-Tibet Plateau. J. Syst. Evol. 2014, 52, 241–249. [Google Scholar] [CrossRef]
- Reed, T.E.; Schindler, D.E.; Waples, R.S. Interacting effects of phenotypic plasticity and evolution on population persistence in a changing climate. Conserv. Biol. 2011, 25, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Bongers, F.J.; Olmo, M.; Lopez-Iglesias, B.; Anten, N.P.R.; Villar, R. Drought responses, phenotypic plasticity and survival of Mediterranean species in two different microclimatic sites. Plant Biol. 2017, 19, 386–395. [Google Scholar] [CrossRef]
- Duputie, A.; Rutschmann, A.; Ronce, O.; Chuine, I. Phenological plasticity will not help all species adapt to climate change. Glob. Change Biol. 2015, 21, 3062–3073. [Google Scholar] [CrossRef]
- Walker, T.W.N.; Weckwerth, W.; Bragazza, L.; Fragner, L.; Forde, B.G.; Ostle, N.J.; Signarbieux, C.; Sun, X.; Ward, S.E.; Bardgett, R.D. Plastic and genetic responses of a common sedge to warming have contrasting effects on carbon cycle processes. Ecol. Lett. 2019, 22, 159–169. [Google Scholar] [CrossRef]
- Kreyling, J.; Puechmaille, S.J.; Malyshev, A.V.; Valladares, F. Phenotypic plasticity closely linked to climate at origin and resulting in increased mortality under warming and frost stress in a common grass. Ecol. Evol. 2019, 9, 1344–1352. [Google Scholar] [CrossRef]
- May, R.-L.; Warner, S.; Wingler, A. Classification of intra-specific variation in plant functional strategies reveals adaptation to climate. Ann. Bot. 2017, 119, 1343–1352. [Google Scholar] [CrossRef]
- Davis, M.B.; Shaw, R.G. Range shifts and adaptive responses to Quaternary climate change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogues-Bravo, D.; Rodriguez-Sanchez, F.; Orsini, L.; de Boer, E.; Jansson, R.; Morlon, H.; Fordham, D.A.; Jackson, S.T. Cracking the code of biodiversity responses to past climate change. Trends Ecol. Evol. 2018, 33, 765–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumstein, M.; Richardson, A.; Weston, D.; Zhang, J.; Muchero, W.; Hopkins, R. A new perspective on ecological prediction reveals limits to climate adaptation in a temperate tree species. Curr. Biol. 2020, 30, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, X.; Li, X.; Zhao, X.; Wu, Z.; Xiao, Y.; Guo, Y. Will the climate of plant origins influence the chemical profiles of cuticular waxes on leaves of Leymus chinensis in a common garden experiment? Ecol. Evol. 2020, 10, 543–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotsch, S.G.; Powers, J.S.; Lerdau, M.T. Leaf traits and water relations of 12 evergreen species in Costa Rican wet and dry forests: Patterns of intra-specific variation across forests and seasons. Plant Ecol. 2010, 211, 133–146. [Google Scholar] [CrossRef]
- Bresta, P.; Nikolopoulos, D.; Stavroulaki, V.; Vahamidis, P.; Economou, G.; Karabourniotis, G. How does long-term drought acclimation modify structure-function relationships? A quantitative approach to leaf phenotypic plasticity of barley. Funct. Plant Biol. 2018, 45, 1181–1194. [Google Scholar] [CrossRef]
- Franks, S.J.; Weber, J.J.; Aitken, S.N. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol. Appl. 2014, 7, 123–139. [Google Scholar] [CrossRef]
- Oliveras, I.; Bentley, L.; Fyllas, N.M.; Gvozdevaite, A.; Shenkin, A.F.; Prepah, T.; Morandi, P.; Peixoto, K.S.; Boakye, M.; Adu-Bredu, S.; et al. The influence of taxonomy and environment on leaf trait variation along tropical abiotic gradients. Front. For. Glob. Chang. 2020, 3. [Google Scholar] [CrossRef] [Green Version]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic-carbon in soil. Commun. Soil Sci. Plan. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Bao, S.D. Agricultural Chemical Analysis of Soil; Agriculture Press: Beijing, China, 2005. [Google Scholar]
- He, D.; Huang, H.; Arismendi, G.G. n-Alkane distribution in ombrotrophic peatlands from the northeastern Alberta, Canada, and its paleoclimatic implications. Palaeogeogr. Palaeocl. 2019, 528, 247–257. [Google Scholar] [CrossRef]
- Poynter, J.; Eglinton, G. Molecular Composition of Three Sediments from Hole 717C: The Bengal Fan. In Proceedings of the Ocean Drilling Program Scientific Results; Texas A & M University: College Station, TX, USA, 1990; Volume 155–161. [Google Scholar]
- Mazurek, M.A.; Simoneit, B.R.T. Higher molecular weight terpenoids as indicators of organic emissions from terrestrial vegetation. In Molecular Markers in Environmental Geochemistry; Eganhouse, R.P., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1997; Volume 671, pp. 92–108. [Google Scholar]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant Physiol. 2000, 27, 595–607. [Google Scholar] [CrossRef]
- Valladares, F.; Wright, S.J.; Lasso, E.; Kitajima, K.; Pearcy, R.W. Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 2000, 81, 1925–1936. [Google Scholar] [CrossRef]






| Year | Plant Species | Site | Transplanting (T) | Y × P | Y × S | Y × T | P × S | P × T | S × T | Y × P × S | Y × P × T | P × S × T | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height | 3.676 ns | 16.310 ** | 219.332 *** | 61.054 *** | 5.243 * | 10.334 * | 0.044 ns | 16.703 *** | 1.313 ns | 11.001 * | 5.509 * | 0.594 ns | 3.741 ns |
| LL | 5.286 ns | 22.351 ** | 33.243 ** | 8.588 * | 1.487 ns | 3.273 ns | 2.651 ns | 5.793 * | 3.007 ns | 0.174 ns | 1.724 ns | 1.239 ns | 6.459 * |
| LW | 16.849 ** | 188.048 *** | 26.282 ** | 14.684 ** | 3.436 ns | 0.173 ns | 3.815 ns | 12.153 ** | 2.095 ns | 5.212 ns | 0.211 ns | 0.958 ns | 3.911 ns |
| LT | 2.053 ns | 30.140 *** | 67.430 *** | 3.967 ns | 1.088 ns | 1.027 ns | 1.496 ns | 14.656 ** | 12.937 ** | 0.011 ns | 0.901 ns | 1.000 ns | 10.264 ** |
| LSA | 8.912 * | 13.274 ** | 0.000 ns | 7.548 * | 0.670 ns | 3.025 ns | 1.730 ns | 2.972 ns | 1.500 ns | 0.537 ns | 4.862 * | 1.538 ns | 4.521 * |
| chlorophyll | 1.547 ns | 3805.702 *** | 1434.214 *** | 876.592 *** | 1.247 ns | 0.346 ns | 1.272 ns | 371.77 *** | 316.469 *** | 1497.712 *** | 1.145 ns | 1.167 ns | 314.742 *** |
| total wax | 2.548 ns | 8.112 * | 6.898 * | 4.348 ns | 5.376 * | 0.032 ns | 0.479 ns | 3.452 ns | 2.531 ns | 1.242 ns | 4.838 * | 2.285 ns | 1.750 ns |
| alkanes | 8.729 * | 9.329 ** | 4.330 ns | 0.966 ns | 3.519 ns | 0.913 ns | 0.018 ns | 2.353 ns | 2.578 ns | 0.902 ns | 2.148 ns | 1.221 ns | 0.705 ns |
| alcohols | 184.844 *** | 1236.322 *** | 82.709 *** | 11.553 * | 91.938 *** | 179.882 *** | 1.215 ns | 53.043 *** | 9.968 ** | 32.439 ** | 221.710 *** | 4.592 * | 37.705 *** |
| acids | 188.534 *** | 111.142 *** | 3.139 ns | 8.719 * | 111.142 *** | 3.139 ns | 8.719 * | 1.331 ns | 6.741 * | 1.068 ns | 1.331 ns | 6.741 * | 0.986 ns |
| ACLalkane | 3.161 ns | 1107.626 *** | 669.139 *** | 879.018 *** | 5.795 * | 1.351 ns | 0.086 ns | 852.105 *** | 818.575 *** | 824.850 *** | 4.497 * | 1.545 ns | 941.012 *** |
| CPIalkane | 10.078 * | 11.481 ** | 17.429 ** | 0.816 ns | 11.619 ** | 12.962 * | 0.015 ns | 8.916 ** | 0.340 ns | 2.409 ns | 10.078 ** | 0.244 ns | 0.633 ns |
| ACLalcohol | 111.223 *** | 30.430 *** | 0.613 ns | 1.225 ns | 22.662 ** | 0.889 ns | 0.984 ns | 1.079 ns | 0.964 ns | 0.896 ns | 1.013 ns | 1.013 ns | 1.041 ns |
| ACLacid | 326.320 *** | 15.474 ** | 0.387 ns | 1.290 ns | 15.474 ** | 0.387 ns | 1.290 ns | 0.994 ns | 0.855 ns | 0.570 ns | 0.994 ns | 0.855 ns | 1.086 ns |
| 2018 | 2019 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant Speices | Indexes | HT/HL | HT/QL | QT/QL | QT/HL | HT/HL | HT/QL | QT/QL | QT/HL |
| P. asiatica | alkanes | 1.061 | 1.423 | 1.236 | 0.922 | 0.867 | 0.872 | 1.219 | 1.212 |
| acids | 2.160 | 4.285 | 1.595 | 0.804 | - | - | - | - | |
| alcohols | 0.776 | 2.185 | 1.419 | 0.504 | - | - | - | - | |
| aldehydes | - | - | - | - | 0.769 | 1.274 | 1.198 | 0.722 | |
| total wax | 0.997 | 1.594 | 1.302 | 0.815 | 0.863 | 0.855 | 1.218 | 1.229 | |
| P. viviparum | alkanes | 1.929 | 0.560 | 0.416 | 1.434 | 2.355 | 1.914 | 1.072 | 1.319 |
| acids | 1.352 | 3.163 | 0.304 | 0.130 | - | - | - | - | |
| alcohols | 1.098 | 4.115 | 2.020 | 0.539 | 0.771 | 1.044 | 1.469 | 1.084 | |
| aldehydes | 0.364 | 0.899 | 0.938 | 0.380 | 1.201 | 0.679 | 0.952 | 1.683 | |
| diketone | 0.640 | 0.583 | 0.210 | 0.230 | 0.880 | 0.447 | 1.084 | 2.132 | |
| total wax | 0.807 | 0.853 | 0.280 | 0.265 | 1.078 | 0.620 | 1.045 | 1.817 | |
| A. lactea | alkanes | 0.642 | 3.189 | 2.515 | 0.506 | 0.725 | 0.065 | 4.710 | 52.847 |
| alcohols | 6.904 | 9.718 | 0.641 | 0.456 | - | - | - | - | |
| esters | - | - | - | - | 1.653 | 0.106 | 13.456 | 209.515 | |
| total wax | 0.314 | 1.391 | 1.032 | 0.233 | 0.741 | 0.070 | 5.483 | 57.968 | |
| K. humilis | alkanes | 0.000 | 0.000 | 1.320 | 0.525 | 0.000 | 0.000 | 0.542 | 0.571 |
| acids | 0.000 | 0.000 | 0.294 | 0.466 | - | - | - | - | |
| alcohols | 0.000 | 0.000 | 0.810 | 1.521 | - | - | - | - | |
| esters | 0.000 | 0.000 | 1.356 | 0.038 | - | - | - | - | |
| aldehydes | - | - | - | - | 0.000 | 0.000 | 0.109 | 0.173 | |
| total wax | 0.000 | 0.000 | 1.033 | 0.478 | 0.000 | 0.000 | 0.454 | 0.564 | |
| L. nanum | alkanes | 0.772 | 0.677 | 0.349 | 0.398 | 0.266 | 0.713 | 0.698 | 0.261 |
| acids | 0.767 | 0.778 | 0.465 | 0.458 | - | - | - | - | |
| alcohols | - | - | - | - | 0.225 | 1.080 | 0.695 | 0.145 | |
| esters | 0.125 | - | - | 0.000 | 0.143 | 0.313 | 0.325 | 0.148 | |
| aldehydes | - | - | - | - | 0.227 | 0.375 | 1.849 | 1.119 | |
| total wax | 0.702 | 0.712 | 0.422 | 0.416 | 0.262 | 0.700 | 0.729 | 0.273 | |
| P. chinensis | alkanes | 0.913 | 2.612 | 0.552 | 0.193 | 1.758 | 8.958 | 1.992 | 0.391 |
| acids | 0.969 | 4.182 | 1.291 | 0.299 | - | - | - | - | |
| alcohols | 1.393 | 0.794 | 0.858 | 1.504 | 1.635 | 9.721 | 0.402 | 0.068 | |
| amyrin | 2.365 | 5.171 | 1.323 | 0.605 | - | - | - | - | |
| aldehydes | 0.686 | 0.802 | 0.410 | 0.351 | 0.990 | 6.774 | 2.186 | 0.320 | |
| total wax | 1.260 | 1.577 | 0.810 | 0.647 | 1.616 | 8.281 | 1.764 | 0.344 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Wang, D.; Wang, D.; Li, S.; Chen, Y.; Guo, Y. Phenotypic Plasticity and Local Adaptation of Leaf Cuticular Waxes Favor Perennial Alpine Herbs under Climate Change. Plants 2022, 11, 120. https://doi.org/10.3390/plants11010120
Yao L, Wang D, Wang D, Li S, Chen Y, Guo Y. Phenotypic Plasticity and Local Adaptation of Leaf Cuticular Waxes Favor Perennial Alpine Herbs under Climate Change. Plants. 2022; 11(1):120. https://doi.org/10.3390/plants11010120
Chicago/Turabian StyleYao, Luhua, Dengke Wang, Dangjun Wang, Shixiong Li, Youjun Chen, and Yanjun Guo. 2022. "Phenotypic Plasticity and Local Adaptation of Leaf Cuticular Waxes Favor Perennial Alpine Herbs under Climate Change" Plants 11, no. 1: 120. https://doi.org/10.3390/plants11010120