Effect of Elevated Temperature on Tomato Post-Harvest Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Tomato Germplasm for Assessment of Post-Harvest Fruit Quality Parameters
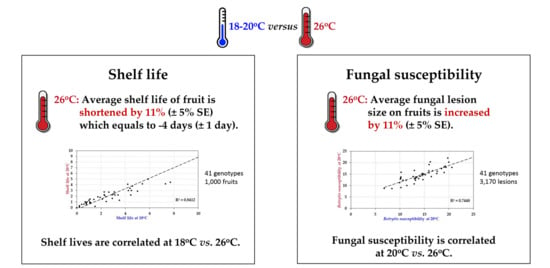
2.2. The Effect of Elevated Temperature on Tomato Shelf-Life Properties
2.3. The Impact of Temperature Increase on Fungal Susceptibility of Tomato Fruit
2.4. Characterisation of Tomato Post-Harvest Properties under Two Temperature Regimes
3. Materials and Methods
3.1. Description of Plant Collection and Material
3.2. Storage Tests
3.3. Fungal Susceptibility Tests
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.-L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-term climate change: Projections, commitments and irreversibility. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 1029–1136. [Google Scholar]
- Li, Y.; Wang, H.; Zhang, Y.; Martin, C. Can the world’s favorite fruit, tomato, provide an effective biosynthetic chassis for high-value metabolites? Plant Cell Rep. 2018, 37, 1443–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsamir, M.; Mahmood, T.; Trethowan, R.; Ahmad, N. An overview of heat stress in tomato (Solanum lycopersicum L.). Saudi. J. Biol. Sci. 2021, 28, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Bertin, N.; Génard, M. Tomato quality as influenced by preharvest factors. Sci. Hortic. 2018, 233, 264–276. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Barrett, D.M.; Garcia, E.; Wayne, J.E. Textural modification of processing tomatoes. Crit. Rev. Food Sci. Nutr. 1998, 38, 173–258. [Google Scholar] [CrossRef]
- Passam, H.C.; Karapanos, I.C.; Bebeli, P.J.; Savvas, D. A review of recent research on tomato nutrition, breeding and post-harvest technology with reference to fruit quality. Eur. J. Plant Sci. Biotechnol. 2007, 1, 1–21. [Google Scholar]
- Giovannoni, J.J.; Nguyen, C.; Ampofo, B.; Zhong, S.; Fei, Z. The epigenome and transcriptional dynamics of fruit ripening. Annu. Rev. Plant Biol. 2017, 68, 61–84. [Google Scholar] [CrossRef]
- Klee, H.J.; Giovannoni, J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Plant Biol. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.B.; Østergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit development and ripening. Annu. Rev. Plant Biol. 2013, 64, 219–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Biol. 2012, 13, 414–430. [Google Scholar]
- Nakajima, M.; Akutsu, K. Virulence factors of Botrytis cinerea. J. Gen. Plant Pathol. 2014, 80, 15–23. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Cantu, D.; Vicente, A.R.; Greve, L.C.; Dewey, F.M.; Bennett, A.B.; Labavitch, J.M.; Powell, A.L. The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea. Proc. Nat. Acad. Sci. USA 2008, 105, 859–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantu, D.; Blanco-Ulate, B.; Yang, L.; Labavitch, J.M.; Bennett, A.B.; Powell, A.L. Ripening-regulated susceptibility of tomato fruit to Botrytis cinerea requires NOR but not RIN or ethylene. Plant Physiol. 2009, 150, 1434–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrasch, S.; Silva, C.J.; Mesquida-Pesci, S.D.; Gallegos, K.; van den Abeele, C.; Papin, V.; Fernandez-Acero, F.J.; Knapp, S.J.; Blanco-Ulate, B. Infection strategies deployed by Botrytis cinerea, Fusarium acuminatum, and Rhizopus stolonifer as a function of tomato fruit ripening stage. Front. Plant Sci. 2019, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.J.; van den Abeele, C.; Ortega-Salazar, I.; Papin, V.; Adaskaveg, J.A.; Wang, D.; Casteel, C.L.; Seymour, G.B.; Blanco-Ulate, B. Host susceptibility factors render ripe tomato fruit vulnerable to fungal disease despite active immune responses. J. Exp. Bot. 2021, 72, 2696–2709. [Google Scholar] [CrossRef]
- Baldwin, E.; Plotto, A.; Narciso, J.; Bai, J. Effect of 1-methylcyclopropene on tomato flavour components, shelf life and decay as influenced by harvest maturity and storage temperature. J. Sci. Food Agric. 2011, 91, 969–980. [Google Scholar] [CrossRef]
- Zhang, B.; Tieman, D.M.; Jiao, C.; Xu, Y.; Chen, K.; Fei, Z.; Giovannoni, J.J.; Klee, H.J. Chilling-induced tomato flavor loss is associated with altered volatile synthesis and transient changes in DNA methylation. Proc. Natl. Acad. Sci. USA 2016, 113, 12580–12585. [Google Scholar] [CrossRef] [Green Version]
- TomGEM: A Holistic Multi-Actor Approach towards the Design of New Tomato Varieties and Management Practices to Improve Yield and Quality in the Face of Climate Change. Available online: https://tomgem.eu/ (accessed on 1 October 2020).
- PhenoTomGEM Database (Phenotyping Database v2.0). Available online: http://tomgem.toulouse.inra.fr (accessed on 1 October 2020).
- Arena, C.; Conti, S.; Francesca, S.; Melchionna, G.; Hájek, J.; Barták, M.; Barone, M.; Rigano, M.M. Eco-physiological screening of different tomato genotypes in response to high temperatures: A combined field-to-laboratory approach. Plants 2020, 9, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balyan, S.; Rao, S.; Jha, S.; Bansal, C.; Das, J.R.; Mathur, S. Characterization of novel regulators for heat stress tolerance in tomato from Indian sub-continent. Plant Biotechnol. J. 2020, 18, 2118–2132. [Google Scholar] [CrossRef] [Green Version]
- Ganeva, D.G.; Grozeva, S.Y.; Pevicharova, G.T. Evaluation of productivity and productivity compounds in tomato accessions grown under elevated temperature and reduced irrigation. Agric. Food 2018, 6, 99–110. [Google Scholar]
- Gonzalo, M.J.; Nájera, I.; Baixauli, C.; Gil, D.; Montoro, T.; Soriano, V.; Olivieri, F.; Rigano, M.M.; Ganeva, D.; Grozeva-Tileva, S.; et al. Identification of tomato accessions as source of new genes for improving heat tolerance: From controlled experiments to field. BMC Plant Biol. 2021, 21, 345. [Google Scholar] [CrossRef]
- Grozeva, S.Y.; Ganeva, D.G.; Pevicharova, G.T. Influence of reduced irrigation on phenological and morphological characters of different tomato genotypes. Agric. Food 2018, 6, 111–121. [Google Scholar]
- Grozeva, S.Y.; Ganeva, D.G.; Pevicharova, G.T. Screening of tomato genotypes for tolerance of reduced irrigation. In Proceedings of the 15th International Conference on Chemical, Agricultural, Biological & Environmental Science, Lisbon, Portugal, 19–21 June 2019; pp. 36–39. [Google Scholar]
- Olivieri, F.; Calafiore, R.; Francesca, S.; Schettini, C.; Chiaiese, P.; Rigano, M.M.; Barone, A. High-throughput genotyping of resilient tomato landraces to detect candidate genes involved in the response to high temperatures. Genes 2020, 11, 626. [Google Scholar] [CrossRef]
- Pevicharova, G.T.; Ganeva, D.G.; Grozeva, S.Y. Impact of water deficit on sensory profile of tomato (Solanum lycopersicum L.) grown under hot summer conditions in Bulgaria. Agric. Food 2018, 6, 122–137. [Google Scholar]
- Pevicharova, G.T.; Ganeva, D.G.; Grozeva, S.Y. Impact of water deficit on nutritional quality of tomatoes. In Proceedings of the 15th International Conference on Chemical, Agricultural, Biological & Environmental Science, Lisbon, Portugal, 19–21 June 2019; pp. 40–44. [Google Scholar]
- Pernice, R.; Parisi, M.; Giordano, I.; Pentangelo, A.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Antioxidants profile of small tomato fruits: Effect of irrigation and industrial process. Sci. Hortic. 2010, 126, 156–163. [Google Scholar] [CrossRef]
- Ruggieri, V.; Calafiore, R.; Schettini, C.; Rigano, M.M.; Olivieri, F.; Frusciante, L.; Barone, A. Exploiting genetic and genomic resources to enhance heat-tolerance in tomato. Agronomy 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Scarano, A.; Olivieri, F.; Gerardi, C.; Liso, M.; Chiesa, M.; Chieppa, M.; Frusciante, L.; Barone, A.; Santino, A.; Rigano, M.M. Selection of tomato landraces with high fruit yield and nutritional quality under elevated temperatures. J. Sci. Food Agric. 2020, 100, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Pevicharova, G.T.; Ganeva, D.G.; Grozeva, S.Y.; Maritsa Vegetable Crops Research Institute, Plovdiv, Bulgaria. Personal communication, 2017.
- Hanson, P.; World Vegetable Center, Shanhua, Tainan, Taiwan. Personal communication, 2021.
- Opeña, R.T.; Green, S.K.; Talekar, N.S.; Chen, J.T. Genetic improvement of tomato adaptability to the tropics: Progress and future prospects. In Tomato and Pepper Production in the Tropics; Green, S.K., Ed.; AVRDC: Shanhua, Taiwan, 1989; pp. 70–85. [Google Scholar]
- Hernould, M.; Delmas, F.; University of Bordeaux, Bordeaux, France. Personal communication, 2017.
- Geethanjali, S.; Chen, K.Y.; Pastrana, D.V.; Wang, J.-F. Development and characterization of tomato SSR markers from genomic sequences of anchored BAC clones on chromosome 6. Euphytica 2010, 173, 85–97. [Google Scholar] [CrossRef]
- Geethanjali, S.; Kadirvel, P.; de la Peña, R.; Rao, E.S.; Wang, J.-F. Development of tomato SSR markers from anchored BAC clones of chromosome 12 and their application for genetic diversity analysis and linkage mapping. Euphytica 2011, 178, 283–295. [Google Scholar] [CrossRef]
- Prasanna, H.C.; Sinha, D.P.; Rai, G.K.; Krishna, R.; Kashyap, S.P.; Singh, N.K.; Singh, M.; Malathi, V.G. Pyramiding Ty-2 and Ty-3 genes for resistance to monopartite and bipartite tomato leaf curl viruses of India. Plant Pathol. 2015, 64, 256–264. [Google Scholar] [CrossRef]
- Dy, K.S.; Ro, S.; Roeurn, S.; Ngoun, S.; Chea, L.; Theam, P.; Lim, S.; Sor, R.; Roeun, M.; Meas, K. Genetic variation in agronomic traits and yield performances of tomato (Solanum lycopersicum) genotypes in response to heat stress. Asian J. Agric. Environ. Saf. 2020, 1, 32–38. [Google Scholar]
- Comlekcioglu, N.; Soylu, M.K.; Olgun, M. Evaluation of tomato genotypes for high temperature tolerance using certain reproductive and fruit traits by factor analysis. Int. J. Agric. For. Life Sci. 2020, 4, 190–196. [Google Scholar]
- Ro, S.; Chea, L.; Ngoun, S.; Stewart, Z.P.; Roeurn, S.; Theam, P.; Lim, S.; Sor, R.; Kosal, M.; Roeun, M.; et al. Response of tomato genotypes under different high temperatures in field and greenhouse conditions. Plants 2021, 10, 449. [Google Scholar] [CrossRef]
- Ayenan, M.A.T.; Danquah, A.; Agre, P.A.; Hanson, P.; Asante, I.K.; Danquah, E.Y. Genomic and phenotypic diversity of cultivated and wild tomatoes with varying levels of heat tolerance. Genes 2021, 12, 503. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, V.; Francese, G.; Sacco, A.; D’Alessandro, A.; Rigano, M.M.; Parisi, M.; Milone, M.; Cardi, T.; Mennella, G.; Barone, A. An association mapping approach to identify favourable alleles for tomato fruit quality breeding. BMC Plant Biol. 2014, 14, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacco, A.; Ruggieri, V.; Parisi, M.; Festa, G.; Rigano, M.M.; Picarella, M.E.; Mazzucato, A.; Barone, A. Exploring a tomato landraces collection for fruit-related traits by the aid of a high-throughput genomic platform. PLoS ONE 2015, 10, e0137139. [Google Scholar] [CrossRef] [Green Version]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of minimal processing on bioactive compounds and color attributes of fresh-cut tomatoes. LWT Food Sci. Technol. 2008, 41, 217–226. [Google Scholar] [CrossRef]
- Oztekin, G.B.; Tuzel, Y. Comparative salinity responses among tomato genotypes and rootstocks. Pak. J. Bot. 2011, 43, 2665–2672. [Google Scholar]
- Xu, J.; Wolters-Ars, M.; Mariani, C.; Huber, H.; Rieu, I. Heat stress affects vegetative and reproductive performance and trait correlations in tomato (Solanum lycopersicum). Euphytica 2017, 213, 156. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Butelli, E.; De Stefano, R.; Schoonbeek, H.-J.; Magusin, A.; Pagliarani, C.; Wellner, N.; Hill, L.; Orzaez, D.; Granell, A.; et al. Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold. Curr. Biol. 2013, 23, 1094–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; De Stefano, R.; Robine, M.; Butelli, E.; Bulling, K.; Hill, L.; Rejzek, M.; Martin, C.; Schoonbeek, H.-J. Different reactive oxygen species scavenging properties of flavonoids determine their abilities to extend the shelf life of tomato. Plant Physiol. 2015, 169, 1568–1583. [Google Scholar]
- Conesa, M.À.; Fullana-Pericàs, M.; Granell, A.; Galmés, J. Mediterranean long shelf-life landraces: An untapped genetic resource for tomato improvement. Front. Plant Sci. 2020, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Statista. Monthly Average Daily Temperatures in the United Kingdom (UK) from 2015 to 2020. Available online: https://www.statista.com/statistics/322658/monthly-average-daily-temperatures-in-the-united-kingdom-uk/ (accessed on 28 January 2021).
- Bangerth, F.; Ho, L.C. Fruit position and fruit set sequence in a truss as factors determining final size of tomato fruits. Ann. Bot. 1984, 53, 315–320. [Google Scholar] [CrossRef]
- Bertin, N. Competition for assimilates and fruit position affect fruit set in indeterminate greenhouse tomato. Ann. Bot. 1995, 75, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Jackman, R.L.; Gibson, H.J.; Stanley, D.W. The effects of chilling on tomato fruit texture. Physiol. Plant. 1992, 86, 600–608. [Google Scholar] [CrossRef]
- Tadesse, T.; Ibrahim, A.; Abtew, W. Degradation and formation of fruit color in tomato (Solanum lycopersicum L.) in response to storage temperature. Am. J. Food Technol. 2015, 10, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Mondal, K.; Sharma, N.S.; Malhotra, S.P.; Dhawan, K.; Singh, R. Oxidative stress and antioxidant systems in tomato fruits during storage. J. Food Biochem. 2003, 27, 515–527. [Google Scholar] [CrossRef]
- Barry, C.S.; Giovannoni, J.J. Ripening in the tomato Green-ripe mutant is inhibited by ectopic expression of a protein that disrupts ethylene signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 7923–7928. [Google Scholar] [CrossRef] [Green Version]
- Bota, J.; Conesa, M.À.; Ochogavia, J.M.; Medrano, H.; Francis, D.M.; Cifre, J. Characterization of a landrace collection for Tomàtiga de Ramellet (Solanum lycopersicum L.) from the Balearic Islands. Genet. Resour. Crop. Evol. 2014, 61, 1131–1146. [Google Scholar] [CrossRef]
- Casals, J.; Pascual, L.; Cañizares, J.; Cebolla-Cornejo, J.; Casañas, F.; Nuez, F. Genetic basis of long shelf life and variability into Penjar tomato. Genet. Resour. Crop. Evol. 2012, 59, 219–229. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Fruit ripening mutants yield insights into ripening control. Curr. Opin. Plant Biol. 2007, 10, 283–289. [Google Scholar] [CrossRef]
- Kopeliovitch, E.; Mizrahi, Y.; Rabinowitch, H.D.; Kedar, N. Physiology of the tomato mutant alcobaca. Physiol. Plant. 1980, 48, 307–311. [Google Scholar] [CrossRef]
- Manning, K.; Tör, M.; Poole, M.; Hong, Y.; Thompson, A.J.; King, G.J.; Giovannoni, J.J.; Seymour, G.B. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat. Genet. 2006, 38, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Orfila, C.; Seymour, G.B.; Willats, W.G.; Huxham, I.M.; Jarvis, M.C.; Dover, C.J.; Thompson, A.J.; Knox, J.P. Altered middle lamella homogalacturonan and disrupted deposition of (1–>5)-α-L-arabinan in the pericarp of Cnr, a ripening mutant of tomato. Plant Physiol. 2001, 126, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Saladié, M.; Matas, A.J.; Isaacson, T.; Jenks, M.A.; Goodwin, S.M.; Niklas, K.J.; Xiaolin, R.; Labavitch, J.M.; Shackel, K.A.; Fernie, A.R.; et al. A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol. 2007, 144, 1012–1028. [Google Scholar] [CrossRef] [Green Version]
- Vrebalov, J.; Ruezinsky, D.; Padmanabhan, V.; White, R.; Medrano, D.; Drake, R.; Schuch, W.; Giovannoni, J. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 2002, 296, 343–346. [Google Scholar] [CrossRef]
- Wilkinson, J.Q.; Lanahan, M.B.; Yen, H.C.; Giovannoni, J.J.; Klee, H.J. An ethylene-inducible component of signal transduction encoded by never-ripe. Science 1995, 270, 1807–1809. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Cheema, D.S.; Pathak, D. Heterosis breeding in tomato involving rin, nor and alc alleles: A review of literature. Adv. Hort. Sci. 2008, 22, 54–62. [Google Scholar]
- Osorio, S.; Carneiro, R.T.; Lytovchenko, A.; McQuinn, R.; Sørensen, I.; Vallarino, J.G.; Giovannoni, J.J.; Fernie, A.R.; Rose, J.K.C. Genetic and metabolic effects of ripening mutations and vine detachment on tomato fruit quality. Plant Biotechnol. J. 2020, 18, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Paran, I.; van der Knaap, E. Genetic and molecular regulation of fruit and plant domestication traits in tomato and pepper. J. Exp. Bot. 2007, 58, 3841–3852. [Google Scholar] [CrossRef] [Green Version]
- Thole, V.; Butelli, E.; Wang, H.; Vain, P.; Martin, C.R. Improvement of tomato post-harvest quality. In Proceedings of the XIV Solanaceae and 3rd Cucurbitaceae Joint Conference, Valencia, Spain, 3–6 September 2017; pp. 92–155. [Google Scholar]
- Thole, V.; Vain, P.; Yang, R.-Y.; Almeida, J.; Enfissi, E.M.A.; Nogueira, M.; Price, E.J.; Alseekh, S.; Fernie, A.R.; Fraser, P.D.; et al. Analysis of tomato post-harvest properties: Fruit colour, shelf life, and fungal susceptibility. Curr. Protoc. Plant Biol. 2020, 5, e20108. [Google Scholar] [CrossRef]
- Maul, F.; Sargent, S.; Sims, C.; Baldwin, E.; Balaban, M.; Huber, D. Tomato flavor and aroma quality as affected by storage temperature. J. Food Sci. 2000, 65, 1228–1237. [Google Scholar] [CrossRef]
- Andreakis, N.; Giordano, I.; Pentangelo, A.; Fogliano, V.; Graziani, G.; Monti, L.M.; Rao, R. DNA fingerprinting and quality traits of Corbarino cherry-like tomato landraces. J. Agric. Food Chem. 2004, 52, 3366–3371. [Google Scholar] [CrossRef]
- Asselbergh, B.; Curvers, K.; França, S.C.; Audenaert, K.; Vuylsteke, M.; Van Breusegem, F.; Höfte, M. Resistance to Botrytis cinerea in sitiens, an abscisic acid-deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiol. 2007, 144, 1863–1877. [Google Scholar] [CrossRef] [Green Version]
- Curvers, K.; Seifi, H.; Mouille, G.; de Rycke, R.; Asselbergh, B.; Van Hecke, A.; Vanderschaeghe, D.; Höfte, H.; Callewaert, N.; Van Breusegem, F.; et al. Abscisic acid deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistance to Botrytis cinerea. Plant Physiol. 2010, 154, 847–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Ulate, B.; Vincenti, E.; Powell, A.; Cantu, D. Tomato transcriptome and mutant analyses suggest a role for plant stress hormones in the interaction between fruit and Botrytis cinerea. Front. Plant Sci. 2013, 4, 142. [Google Scholar] [CrossRef] [Green Version]
- Isaacson, T.; Kosma, D.K.; Matas, A.J.; Buda, G.J.; He, Y.; Yu, B.; Pravitasari, A.; Batteas, J.D.; Stark, R.E.; Jenks, M.A.; et al. Cutin deficiency in the tomato fruit cuticle consistently affects resistance to microbial infection and biomechanical properties, but not transpirational water loss. Plant J. 2009, 60, 363–377. [Google Scholar] [CrossRef]
- Bassolino, L.; Zhang, Y.; Schoonbeek, H.-J.; Kiferle, C.; Perata, P.; Martin, C. Accumulation of anthocyanins in tomato skin extends shelf life. New Phytol. 2013, 200, 650–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petric, T.; Kiferle, C.; Perata, P.; Gonzali, S. Optimizing shelf life conditions for anthocyanin-rich tomatoes. PLoS ONE 2018, 13, e0205650. [Google Scholar]
- Szymański, J.; Bocobza, S.; Panda, S.; Sonawane, P.; Cárdenas, P.D.; Lashbrooke, J.; Kamble, A.; Shahaf, N.; Meir, S.; Bovy, A.; et al. Analysis of wild tomato introgression lines elucidates the genetic basis of transcriptome and metabolome variation underlying fruit traits and pathogen response. Nat. Genet. 2020, 52, 1111–1121. [Google Scholar] [CrossRef]
- Charles, M.T.; Goulet, A.; Arul, J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit. IV. Biochemical modification of structural barriers. Postharvest Biol. Technol. 2008, 47, 41–53. [Google Scholar] [CrossRef]
- Charles, M.T.; Mercier, J.; Makhlouf, J.; Arul, J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit. I. Role of pre- and post-challenge accumulation of the phytoalexin rishitin. Postharvest Biol. Technol. 2008, 47, 10–20. [Google Scholar] [CrossRef]
- Charles, M.T.; Tano, K.; Asselin, A.; Arul, J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit. V. Constitutive defence enzymes and inducible pathogenesis-related proteins. Postharvest Biol. Technol. 2009, 51, 414–424. [Google Scholar] [CrossRef]
- Lavelli, V. Circular food supply chains—Impact on value addition and safety. Trends Food Sci. Technol. 2021, 114, 323–332. [Google Scholar] [CrossRef]





| Accession Identifiers (Code/Name) | Growth Habit | Type of Commercial Use | Origin | Fruit Size a | Thermo- tolerance b | Reference c |
|---|---|---|---|---|---|---|
| BG Alia | indeterminate | fresh market | Bulgaria | very small (5–15 g) | HT | [26,31] |
| BG K3 | determinate | processing | Bulgaria | medium-large (75–85 g) | HT | [31] |
| BG Marti | determinate | fresh market and processing | Bulgaria | medium-large (75–90 g) | HT | [25,30,31] |
| BG Solaris | determinate | fresh market and processing | Bulgaria | large (105–125 g) | HS | [27,28,30] |
| BG 10β/14 | determinate | processing | Bulgaria | medium (60–70 g) | HT | [31] |
| BG 11/15 | determinate | processing | Bulgaria | medium (50–60 g) | HT | [31] |
| BG 24/a | indeterminate | fresh market and processing | Bulgaria | medium (30–40 g) | HS | [35] |
| BG 617/14 | indeterminate | fresh market | Bulgaria | very large (230–285 g) | HT | [26,31] |
| BG 895/15 | determinate | processing | Bulgaria | medium (45–55 g) | HT | [31] |
| BG 1620/15 | determinate | fresh market | Bulgaria | very small (5–10 g) | HT | [23,31] |
| BG 1923/15 | indeterminate | fresh market | Bulgaria | very small (5–10 g) | HT | [26,31] |
| BG 2081/15 | determinate | processing | Bulgaria | medium-large (95–105 g) | HT | [31] |
| BL1146/Siberia | determinate | fresh market | Russia | medium (40–50 g) | HT | [36] * |
| BL1350/Tomato337 | indeterminate | fresh market | Nigeria | small (15–25 g) | HT | [36] |
| BL1827/Divisoria-2 | indeterminate | fresh market | Philippines | small (20–25 g) | HT | [37] |
| Brioso RZ ** | indeterminate | fresh market | Rijk Zwaan, France | small (20–30 g) | HT | [38] |
| CA4 | semi-determinate | fresh market | Israel | medium-large (70–90 g) | HS | [24,39,40,41] |
| CLN1621L | determinate | fresh market | Taiwan | small (30–35 g) | HT | [24,39,40,42,43,44,45] |
| Clodano ** | indeterminate | fresh market | Syngenta, France | medium (40–50 g) | HS | [38] |
| E7/Corbarino PC04 | indeterminate | processing | Italy | small (20–30 g) | HT | [23,26,29,33,34,46,47] |
| E8/Corbarino PC05 | indeterminate | processing | Italy | small (25–35 g) | HT | [23,26,29,32,33,34,46,47] |
| E17/Pantano Romanesco | indeterminate | fresh market | Italy | large (160–195 g) | HT | [23,26,29,33,34,46,47] |
| E30/Corbarino PC07 | indeterminate | processing | Italy | small (25–35 g) | HS | [33,46,47] |
| E36/Vesuvio Foglia Riccia San Vito | indeterminate | fresh market and processing | Italy | small (25–35 g) | HT | [23,26,29,33, 34] |
| E37/Siccagno | indeterminate | mainly fresh market | Italy | small (25–30 g) | HT | [23,26,29,33,47] |
| E41/Parmitanella | semi-determinate | fresh market | Italy | small-medium (20–30 g) | HS | [23,33,46,47] |
| E42/PI15250 | determinate | potentially fresh market and processing | Italy | small (15–20 g) | HT | [23,29,33] |
| E45/SM246 | indeterminate | processing | Italy | medium (50–55 g) | HT | [23,26,29,33, 46,47] |
| E53/LA0147 | indeterminate | fresh market | Honduras | medium-large (80–95 g) | HT | [23,26,29,33, 34,47] |
| E76/LA4449/Black Plum | indeterminate | processing | Russia | small (25–30 g) | HT | [23,26,29,33, 34,46,47] |
| E107/EA06462/ E-L-19 | semi-determinate | fresh market | Spain | medium (65–75 g) | HT | [23,29,33,46, 47] |
| Docet ** | determinate | processing | Monsanto, Italy | medium (55–65 g) | HT | [26,29] |
| Durinta ** | indeterminate | fresh market | Western Seed International BV, The Netherlands | medium-large (65–80 g) | HT | [26,48,49] |
| JAG8810 ** | determinate | processing | Monsanto, Italy | medium-large (70–80 g) | HT | [23,25,26,29] |
| Manadi ** | indeterminate | fresh market | Enza Zaden, The Netherlands | medium-large (95–115 g) | HT | [26] |
| M82/LA3475 ** | determinate | processing | Israel | medium (55–60 g) | moderate HT | [23,26,33] |
| MoneyMaker (MM)/LA2706 ** | indeterminate | fresh market | United Kingdom | medium-large (70–80 g) | HS | [26,34,46] |
| Monterrey ** | indeterminate | fresh market | Nunhems BV, The Netherlands | very small (5–10 g) | HT | [26] |
| PaiPai ** | indeterminate | fresh market and processing | Enza Zaden, The Netherlands | medium-large (90–105 g) | HT | [26] |
| Temptation** | indeterminate | fresh market | Enza Zaden, The Netherlands | medium (50–55 g) | HT | [26] |
| WVa106/West Virginia 106 *** | indeterminate | fresh market | France | very small (3–7 g) | HT | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thole, V.; Vain, P.; Martin, C. Effect of Elevated Temperature on Tomato Post-Harvest Properties. Plants 2021, 10, 2359. https://doi.org/10.3390/plants10112359
Thole V, Vain P, Martin C. Effect of Elevated Temperature on Tomato Post-Harvest Properties. Plants. 2021; 10(11):2359. https://doi.org/10.3390/plants10112359
Chicago/Turabian StyleThole, Vera, Philippe Vain, and Cathie Martin. 2021. "Effect of Elevated Temperature on Tomato Post-Harvest Properties" Plants 10, no. 11: 2359. https://doi.org/10.3390/plants10112359