Light Spectrum Differentially Affects the Yield and Phytochemical Content of Microgreen Vegetables in a Plant Factory
Abstract
:1. Introduction
2. Results
2.1. Morphology
2.2. Total Soluble Solids
2.3. Phytochemical Analyses
2.4. Chlorophyll and Carotenoid Content
3. Discussion
3.1. Morphology
3.2. Total Soluble Solids
3.3. Phytochemical Analyses
3.4. Chlorophyll and Carotenoid Content
4. Materials and Methods
4.1. Plant Material and Sowing
4.2. Growth and Light Conditions
4.3. Measurements and Analyses
4.3.1. Morphology
4.3.2. Total Soluble Solids (Brix)
4.3.3. Phytochemical Analysis
4.3.4. Chlorophyll and Carotenoid Content
4.3.5. Experimental Design and Statistical Analysis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozai, T.; Niu, G. Role of the plant factory with artificial lighting (PFAL) in urban areas. In Plant Factory; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: London, UK, 2020; pp. 7–34. [Google Scholar]
- Takagaki, M.; Hara, H.; Kozai, T. Micro- and mini-PFALs for improving the quality of life in urban areas. In Plant Factory; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: London, UK, 2020; pp. 117–128. [Google Scholar]
- Folta, K.M.; Childers, K.S. Light as a growth regulator: Controlling plant biology with narrow-bandwidth solid-state lighting systems. HortScience 2008, 43, 1957–1964. [Google Scholar] [CrossRef] [Green Version]
- Whitelam, G.; Halliday, K. Light and Plant Development; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar]
- Sager, J.C.; McFarlane, J.C. Plant growth chamber handbook, Radiation. In Iowa Agriculture and Home Economics Experimental Station Special Report No. 99; Langhans, R.W., Tibbits, T.W., Eds.; Iowa State University Press: Ames, IA, USA, 1997; pp. 1–29. [Google Scholar]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Koukounaras, A.; Siomos, A.S.; Sfakiotakis, E. Postharvest CO2 and ethylene production and quality of rocket (Eruca sativa Mill.) leaves as affected by leaf age and storage temperature. Posthar. Biol. Technol. 2007, 46, 167–173. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G. Plant responses to light. In Plant Factory; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: London, UK, 2020; pp. 153–166. [Google Scholar]
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Sci. Hortic. 2016, 198, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Ouzounis, T.; Parjikolaei, B.R.; Frette, X.; Rosenqvist, E.; Ottosen, C.-O. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Craver, J.K.; Gerovac, J.R.; Lopez, R.G.; Kopsell, D.A. Light intensity and light quality from sole-source light-emitting diodes impact phytochemical concentrations within Brassica microgreens. J. Amer. Soc. Hort. Sci. 2017, 142, 3–12. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Comp. Analys. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current review of the modulatory effects of LED lights on photosynthesis of secondary metabolites and future perspectives of microgreen vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef]
- Nishimura, T.; Zobayed, S.M.; Kozai, T.; Goto, E. Medicinally important secondary metabolites and growth of Hypericum perforatum L. plants as affected by light quality and intensity. Environ. Control. Bio. 2007, 45, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postahrv. Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutrit. 2010, 50, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zheng, Y. Growth and morphology responses to narrow-band blue light and its coaction with low-level UVB or green light: A comparison with red light in four microgreen species. Environ. Exp. Bot. 2020, 178, 104189. [Google Scholar] [CrossRef]
- Brazaityte, A.; Miliauskiene, J.; Vaštakaite-Kairiene, V.; Sutuliene, R.; Laužike, K.; Duchovskis, P.; Małek, S. Effect of different ratios of blue and red LED light on Brassicaceae Microgreens under a controlled environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Ferrón-Carrillo, F.; Guil-Guerrero, J.L.; Gonzalez-Fernandez, M.J.; Lyashenko, S.; Battafarano, F.; da Cunha-Chiamolera, T.P.L.; Urrestarazu, M. LED Enhances Plant Performance and Both Carotenoids and Nitrates Profiles in Lettuce. Plant Food Human Nutrit. 2021, 76, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Su, J.; Liu, B.; Liao, J.; Yang, Z.; Lin, C.; Oka, Y. Coordination of cryptochrome and phytochrome signals in the regulation of plant light responses. Agronomy 2017, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.; Stasiak, M.; Dixon, M.A.; Zheng, Y. Blue light associated with low phytochrome activity can promote elongation growth as shade-avoidance response: A comparison with red light in four bedding plant species. Environ. Exp. Bot. 2018, 155, 345–359. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations—Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Mishra, S.; Khurana, J.P. Emerging roles and new paradigms in signaling mechanisms of plant cryptochromes. Crit. Rev. Plant Sci. 2017, 36, 89–115. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Metereol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the role of red:blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maina, S.; Ryu, D.H.; Cho, J.Y.; Jung, D.S.; Park, J.-E.; Nho, C.W.; Bakari, G.; Misinzo, G.; Jung, J.H.; Yang, S.-H.; et al. Exposure to salinity and light spectra regulates glucosinolates, phenolics, and antioxidant capacity of Brassica carinata L. microgreens. Antioxidants 2021, 10, 1183. [Google Scholar] [CrossRef]
- Gerovac, J.R.; Craver, J.K.; Boldt, J.K.; Lopez, R.G. Light intensity and quality from sole-source light-emitting diodes impact growth, morphology, and nutrient content of Brassica microgreens. HortScience 2016, 51, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Parrish, C.H.; Hebert, D.; Jackson, A.; Ramasamy, K.; McDaniel, H.; Giacomelli, G.A.; Bergren, M.R. Optimizing spectral quality with quantum dots to enhance crop yield in controlled environments. Commun. Biol. 2021, 4, 1124. [Google Scholar] [CrossRef] [PubMed]
- Brazaityte, A.; Duchovskis, P.; Urbonaviciute, A.; Samuoliene, G.; Jankauskiene, J.; Sakalauskaite, J.; Sabajeviene, G.; Sirtautas, R.; Novickovas, A. The effect of light-emitting diodes lighting on the growth of tomato transplants. Zembir. Agric. 2010, 97, 89–98. [Google Scholar]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Bantis, F.; Fotelli, M.; Ilic, Z.S.; Koukounaras, A. Physiological and phytochemical responses of spinach baby leaves grown in a PFAL system with LEDs and saline nutrient solution. Agriculture 2020, 10, 574. [Google Scholar] [CrossRef]
- Chen, X.; Xue, X.; Guo, W.; Wang, L.; Qiao, X. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Długosz-Grochowska, O.; Kołton, A.; Zupnik, M. Effects of LED supplemental lighting on yield and some quality parameters of lamb’s lettuce grown in two winter cycles. Sci. Hortic. 2015, 187, 80–86. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Trivandrum, India, 2006; pp. 23–67. [Google Scholar]
- Kopsell, D.A.; Kopsell, D.E.; Lefsrud, M.G.; Curran-Celentano, J.; Dukach, L.E. Variation in lutein, β-carotene, and chlorophyll concentrations among Brassica oleracea cultigens and seasons. HortScience 2004, 39, 361–364. [Google Scholar] [CrossRef]
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and opportunities of light-emitting diode (LED) as key to modulate antioxidant compounds in plants. A review. Antioxidants 2020, 10, 42. [Google Scholar] [CrossRef]
- Giménez, A.; Martínez-Ballesta, M.d.C.; Egea-Gilabert, C.; Gómez, P.A.; Artés-Hernández, F.; Pennisi, G.; Orsini, F.; Crepaldi, A.; Fernández, J.A. Combined effect of salinity and LED lights on the yield and quality of purslane (Portulaca oleracea L.) microgreens. Horticulturae 2021, 7, 180. [Google Scholar] [CrossRef]
- Lister, C.E.; Lancaster, J.E.; Walker, J.R.I. Phenylalanine Ammonia-lyase (PAL) activity and its relationship to anthocyanin and flavonoid levels in New Zealand-grown apple cultivars. J. Amer. Soc. Hort. Sci. 1996, 121, 281–285. [Google Scholar] [CrossRef]
- Lobiuc, A. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuoliene, G.; Brazaityte, A.; Sirtautas, R.; Virsile, A.; Sakalauskaite, J.; Sakalauskiene, S.; Duchovskis, P. LED illumination affects bioactive compounds in romaine baby leaf lettuce. J. Sci. Food Agric. 2013, 93, 3286–3291. [Google Scholar] [CrossRef] [PubMed]
- Stutte, G.W. Light-emitting diodes for manipulating the phytochrome apparatus. HortScience 2009, 44, 231–234. [Google Scholar] [CrossRef]
- Fan, X.X.; Zang, J.; Xu, Z.G.; Guo, S.R.; Jiao, X.L.; Liu, X.Y.; Gao, Y. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.). Acta Physiol. Plant. 2013, 35, 2721–2726. [Google Scholar] [CrossRef]
- Ruyter, G. Effects of blue-light on pyruvate kinase activity during chloroplast development of unicellular green algae. Photochem. Photobiol. 1982, 35, 229–231. [Google Scholar] [CrossRef]
- Toscano, S.; Cavallaro, V.; Ferrante, A.; Romano, D.; Patané, C. Effects of different light spectra on final biomass production and nutritional quality of two microgreens. Plants 2021, 10, 1584. [Google Scholar] [CrossRef]
- Ying, Q.; Jones-Baumgardt, C.; Zheng, Y.; Bozzo, G. The proportion of blue light from light-emitting diodes alters microgreen phytochemical profiles in a species-specific manner. HortScience 2021, 56, 13–20. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Pilar Cano, M.; Graça Dias, M.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents and research needs. Crit. Rev. Food Sci. Nutrit. 2020, 1–51. [Google Scholar] [CrossRef]
- Samuoliene, G.; Virsile, A.; Brazaityte, A.; Jankauskiene, J.; Sakalauskiene, S.; Vastakaite, V.; Novickovas, A.; Viskeliene, A.; Sasnauskas, A.; Duchovskis, P. Blue light dosage affects carotenoids and tocopherols in microgreens. Food. Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil, 2nd ed.; Circular, California Agricultural Experiment Station: Davis, CA, USA, 1950; p. 347. [Google Scholar]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic Efficiency and Phytochrome Photoequilibria Determination Using Spectral Data. Trans ASAE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Voorrips, R.R.; Steenhuis-Broers, G.; Tiemens-Hulscher, M.; van Bueren, E.T.L. Earliness, leaf surface wax and sugar content predict varietal differences for thrips damage in cabbage. Acta Hortic. 2008, 867, 16. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]







| Microgreen | Sowing Density * | End of Dark Period (d) | Harvest (d) |
|---|---|---|---|
| Mustard | 300 | 3 | 8 |
| Radish | 250 | 3 | 8 |
| Green basil | 600 | 4 | 12 |
| Red amaranth | 1500 | 4 | 12 |
| Garlic chives | 500 | 4 | 12 |
| Borage | 80 | 4 | 12 |
| Pea shoots | 12 | 3 | 10 |
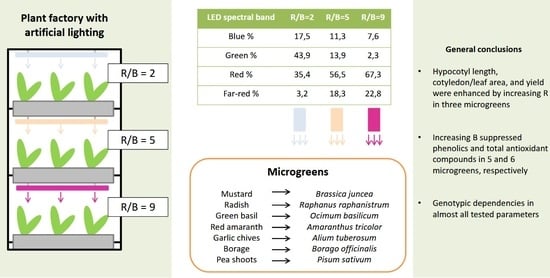
| Parameters | Light Treatment | ||
|---|---|---|---|
| RB2 | RB5 | RB9 | |
| UV %; 380–399 nm | 0.03 | 0.02 | 0.02 |
| Blue %; 400–499 nm | 17.50 | 11.38 | 7.62 |
| Green %; 500–599 nm | 43.84 | 13.85 | 2.34 |
| Red %; 600–699 nm | 35.40 | 56.48 | 67.25 |
| Far-red %; 700–780 nm | 3.23 | 18.28 | 22.77 |
| Blue peak (nm) | 454 | 448 | 448 |
| Red peak (nm) | 600 | 660 | 660 |
| Red/Blue ratio | 2.02 | 4.97 | 8.82 |
| Red/Far-red ratio | 6.44 | 3.09 | 2.95 |
| PPFD (μmol m−2 s−1) | 180 ± 10 | 180 ± 10 | 180 ± 10 |
| YPFD (μmol m−2 s−1) | 84.8 | 75.4 | 73.8 |
| CCT (K) | 4105 | 2143 | - |
| CRI | 85.5 | 71.0 | - |
| PPS | 0.83 | 0.82 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bantis, F. Light Spectrum Differentially Affects the Yield and Phytochemical Content of Microgreen Vegetables in a Plant Factory. Plants 2021, 10, 2182. https://doi.org/10.3390/plants10102182
Bantis F. Light Spectrum Differentially Affects the Yield and Phytochemical Content of Microgreen Vegetables in a Plant Factory. Plants. 2021; 10(10):2182. https://doi.org/10.3390/plants10102182
Chicago/Turabian StyleBantis, Filippos. 2021. "Light Spectrum Differentially Affects the Yield and Phytochemical Content of Microgreen Vegetables in a Plant Factory" Plants 10, no. 10: 2182. https://doi.org/10.3390/plants10102182