Chitosan-Based Nanoparticles with Optimized Parameters for Targeted Delivery of a Specific Anticancer Drug—A Comprehensive Review
Abstract
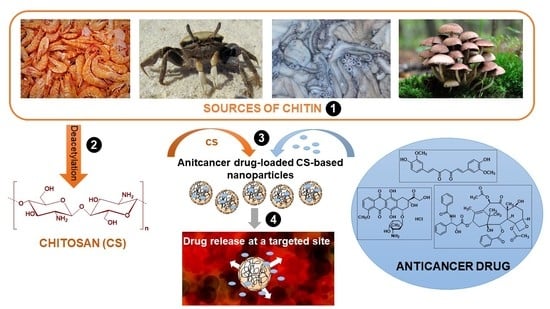
:1. Introduction
1.1. Structure and Properties of Chitosan
1.2. Drug Delivery Systems Based on Chitosan
2. CS NP Platforms for a Specific Drug Delivery
2.1. Doxorubicin as a Representative of Antibiotics
2.2. Antimetabolites
2.2.1. 5-Fluorouracil
2.2.2. Other Antimetabolites
2.3. Platinum Complexes
2.4. Microtubule Damaging Agents
2.5. Drugs Affecting the Hormonal Environment
2.6. Potential Anticancer Agents
2.6.1. Encapsulation of Polyphenols
2.6.2. Encapsulation of Multicomponent Plant Extracts
| Type of Modification/Functionalization | Loaded Drug | Carrier Advantages | Reference |
|---|---|---|---|
| Phenylboronic acid | CUR | High loading Efficient release | [91] |
| Succinic anhydride Mannose | CUR | Enhanced antitumor properties Improved targeting | [93] |
| Iron(II, III) oxide Hyperthermia | CUR | Improved cytotoxicity | [95] |
| Cetuximab | QRT and PTX | Improved targeting Sensitizing drug-resistant cancer cells to PTX | [98] |
| Poly(lactic-co-glycolic acid) Folic acid | EOs | Improved stability Enhanced antitumor properties Improved targeting | [113] |
| Poly(lactic-co-glycolic acid) Folic acid | Smoke extract | Improved stability Enhanced antitumor properties Improved targeting | [114] |
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurczewska, J. Recent Reports on Polysaccharide-Based Materials for Drug Delivery. Polymers 2022, 14, 4189. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Mehta, M.; Satija, S.; Aljabali, A.A.; Tambuwala, M.M.; Anand, K.; Sharma, N.; Dureja, H.; Jha, N.K.; et al. Current-status and applications of polysaccharides in drug delivery systems. Colloid Interface Sci. Commun. 2021, 42, 100418. [Google Scholar] [CrossRef]
- Sun, Y.; Jing, X.; Ma, X.; Feng, Y.; Hu, H. Versatile Types of Polysaccharide-Based Drug Delivery Systems: From Strategic Design to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9159. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.V.S.; Sharma, J.; Kumar, S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef]
- Bastiaens, L.; Soetemans, L.; D’Hondt, E.; Elst, K. Sources of Chitin and Chitosan and Their Isolation. In Chitin and Chitosan; Broek, L.A.M., Boeriu, C.G., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 1–34. ISBN 978-1-119-45043-6. [Google Scholar]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef]
- Budishevska, O.; Popadyuk, N.; Musyanovych, A.; Kohut, A.; Donchak, V.; Voronov, A.; Voronov, S. Formation of Three-Dimensional Polymer Structures through Radical and Ionic Reactions of Peroxychitosan. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 64, pp. 365–390. ISBN 978-0-12-817903-1. [Google Scholar]
- Narmani, A.; Jafari, S.M. Chitosan-based nanodelivery systems for cancer therapy: Recent advances. Carbohydr. Polym. 2021, 272, 118464. [Google Scholar] [CrossRef]
- Baharlouei, P.; Rahman, A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Mar. Drugs 2022, 20, 460. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y. Recent advances of chitosan-based nanoparticles for biomedical and biotechnological applications. Int. J. Biol. Macromol. 2022, 203, 379–388. [Google Scholar] [CrossRef]
- Alhodieb, F.S.; Barkat, A.; Barkat, H.A.; Ab Hadi, H.; Khan, M.I.; Ashfaq, F.; Rahman, M.A.; Hassan, M.Z.; Alanezi, A.A. Chitosan-modified nanocarriers as carriers for anticancer drug delivery: Promises and hurdles. Int. J. Biol. Macromol. 2022, 217, 457–469. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, Y.; Wang, T.; Sun, M.; Chen, X. Nanomaterials as Smart Immunomodulator Delivery System for Enhanced Cancer Therapy. ACS Biomater. Sci. Eng. 2020, 6, 4774–4798. [Google Scholar] [CrossRef]
- Iacob, A.; Lupascu, F.; Apotrosoaei, M.; Vasincu, I.; Tauser, R.; Lupascu, D.; Giusca, S.; Caruntu, I.-D.; Profire, L. Recent Biomedical Approaches for Chitosan Based Materials as Drug Delivery Nanocarriers. Pharmaceutics 2021, 13, 587. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.M.; Rather, G.A.; Patrício, A.; Haq, Z.; Sheikh, A.A.; Shah, M.Z.U.H.; Singh, H.; Alam Khan, A.; Imtiyaz, S.; Ahmad, S.B.; et al. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.; Muchtaridi, M. Chitosan-Based Nanoparticles of Targeted Drug Delivery System in Breast Cancer Treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; Edison, T.N.J.I.; LewisOscar, F.; Kumar, P.; Shanmugam, S.; Pugazhendhi, A. Chitosan nanopolymers: An overview of drug delivery against cancer. Int. J. Biol. Macromol. 2019, 130, 727–736. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yagi, K.; Iwakawa, S.; Hirai, M. Chitosan Induces Apoptosis via Caspase-3 Activation in Bladder Tumor Cells. Jpn. J. Cancer Res. 2001, 92, 459–466. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Rizwanullah, M.; Ahmad, J.; Alasmary, M.Y.; Akhter, M.H.; Abdel-Wahab, B.A.; Warsi, M.H.; Haque, A. Progress in nanomedicine-based drug delivery in designing of chitosan nanoparticles for cancer therapy. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 602–623. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 318. [Google Scholar] [CrossRef]
- Dubey, S.K.; Bhatt, T.; Agrawal, M.; Saha, R.N.; Saraf, S.; Saraf, S.; Alexander, A. Application of chitosan modified nanocarriers in breast cancer. Int. J. Biol. Macromol. 2022, 194, 521–538. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Tavakol, S.; Moghassemi, S.; Dadashzadeh, A.; Schneible, J.D.; Fatemi, I.; Shirvani, A.; Zarrabi, A.; Azedi, F.; Dehshahri, A.; et al. Chitosan: A versatile bio-platform for breast cancer theranostics. J. Control. Release 2022, 341, 733–752. [Google Scholar] [CrossRef]
- Kuen, C.Y.; Masarudin, M.J. Chitosan Nanoparticle-Based System: A New Insight into the Promising Controlled Release System for Lung Cancer Treatment. Molecules 2022, 27, 473. [Google Scholar] [CrossRef]
- Choukaife, H.; Seyam, S.; Alallam, B.; Doolaanea, A.A.; Alfatama, M. Current Advances in Chitosan Nanoparticles Based Oral Drug Delivery for Colorectal Cancer Treatment. Int. J. Nanomed. 2022, 17, 3933–3966. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Hushmandi, K.; Mirzaei, S.; Bokaie, S.; Bigham, A.; Makvandi, P.; Rabiee, N.; Thakur, V.K.; Kumar, A.P.; Sharifi, E.; et al. Chitosan-based nanoscale systems for doxorubicin delivery: Exploring biomedical application in cancer therapy. Bioeng. Transl. Med. 2022, 8, e10325. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Chitosan-based nanocarriers for encapsulation and delivery of curcumin: A review. Int. J. Biol. Macromol. 2021, 179, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.-G.; Chen, T. Antibiotics for cancer treatment: A double-edged sword. J. Cancer 2020, 11, 5135–5149. [Google Scholar] [CrossRef] [PubMed]
- Langevin, P.B.; Atlee, J.L. Chemotherapeutic Agents. In Complications in Anesthesia; Elsevier: Amsterdam, The Netherlands, 2007; pp. 110–118. ISBN 978-1-4160-2215-2. [Google Scholar]
- Helmi, O.; Elshishiny, F.; Mamdouh, W. Targeted doxorubicin delivery and release within breast cancer environment using PEGylated chitosan nanoparticles labeled with monoclonal antibodies. Int. J. Biol. Macromol. 2021, 184, 325–338. [Google Scholar] [CrossRef]
- Choi, Y.; Han, H.; Jeon, S.; Yoon, H.Y.; Kim, H.; Kwon, I.C.; Kim, K. Deep Tumor Penetration of Doxorubicin-Loaded Glycol Chitosan Nanoparticles Using High-Intensity Focused Ultrasound. Pharmaceutics 2020, 12, 974. [Google Scholar] [CrossRef]
- Nogueira-Librelotto, D.R.; Scheeren, L.E.; Macedo, L.B.; Vinardell, M.P.; Rolim, C.M. pH-Sensitive chitosan-tripolyphosphate nanoparticles increase doxorubicin-induced growth inhibition of cervical HeLa tumor cells by apoptosis and cell cycle modulation. Colloids Surf. B 2020, 190, 110897. [Google Scholar] [CrossRef]
- Huang, S.-J.; Wang, T.-H.; Chou, Y.-H.; Wang, H.-M.D.; Hsu, T.-C.; Yow, J.-L.; Tzang, B.-S.; Chiang, W.-H. Hybrid PEGylated chitosan/PLGA nanoparticles designed as pH-responsive vehicles to promote intracellular drug delivery and cancer chemotherapy. Int. J. Biol. Macromol. 2022, 210, 565–578. [Google Scholar] [CrossRef]
- Peng, H.; Qiao, L.; Shan, G.; Gao, M.; Zhang, R.; Yi, X.; He, X. Stepwise responsive carboxymethyl chitosan-based nanoplatform for effective drug-resistant breast cancer suppression. Carbohydr. Polym. 2022, 291, 119554. [Google Scholar] [CrossRef]
- Xu, X.; Xue, Y.; Fang, Q.; Qiao, Z.; Liu, S.; Wang, X.; Tang, R. Hybrid nanoparticles based on ortho ester-modified pluronic L61 and chitosan for efficient doxorubicin delivery. Int. J. Biol. Macromol. 2021, 183, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, H.; Yu, W.; Xiong, X.; Krastev, R.; Ma, X. Preparation of Cationic Amphiphilic Nanoparticles with Modified Chitosan Derivatives for Doxorubicin Delivery. Materials 2021, 14, 7010. [Google Scholar] [CrossRef]
- Yi, G.; Ling, J.; Jiang, Y.; Lu, Y.; Yang, L.-Y.; Ouyang, X. Fabrication, characterization, and in vitro evaluation of doxorubicin-coupled chitosan oligosaccharide nanoparticles. J. Mol. Struct. 2022, 1268, 133688. [Google Scholar] [CrossRef]
- Mi, Y.; Chen, Y.; Gu, G.; Miao, Q.; Tan, W.; Li, Q.; Guo, Z. New synthetic adriamycin-incorporated chitosan nanoparticles with enhanced antioxidant, antitumor activities and pH-sensitive drug release. Carbohydr. Polym. 2021, 273, 118623. [Google Scholar] [CrossRef] [PubMed]
- Amiryaghoubi, N.; Abdolahinia, E.D.; Nakhlband, A.; Aslzad, S.; Fathi, M.; Barar, J.; Omidi, Y. Smart chitosan–folate hybrid magnetic nanoparticles for targeted delivery of doxorubicin to osteosarcoma cells. Colloids Surf. B 2022, 220, 112911. [Google Scholar] [CrossRef]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Patrojanasophon, P. Catechol-modified chitosan/hyaluronic acid nanoparticles as a new avenue for local delivery of doxorubicin to oral cancer cells. Colloids Surf. B 2020, 196, 111279. [Google Scholar] [CrossRef]
- Kong, F.; Tang, C.; Yin, C. Benzylguanidine and Galactose Double-Conjugated Chitosan Nanoparticles with Reduction Responsiveness for Targeted Delivery of Doxorubicin to CXCR 4 Positive Tumors. Bioconjugate Chem. 2020, 31, 2446–2455. [Google Scholar] [CrossRef]
- Momin, T.; Gulbake, A. Development and characterization of doxorubicin and sirna encapsulated chitosan nanoparticles. Int. J. Appl. Pharm. 2020, 12, 53–56. [Google Scholar] [CrossRef]
- Khesht, A.M.S.; Karpisheh, V.; Gilan, P.S.; Melnikova, L.A.; Zekiy, A.O.; Mohammadi, M.; Hojjat-Farsangi, M.; Zolbanin, N.M.; Mahmoodpoor, A.; Hassannia, H.; et al. Blockade of CD73 using siRNA loaded chitosan lactate nanoparticles functionalized with TAT-hyaluronate enhances doxorubicin mediated cytotoxicity in cancer cells both in vitro and in vivo. Int. J. Biol. Macromol. 2021, 186, 849–863. [Google Scholar] [CrossRef]
- Li, Q.; Lv, X.; Tang, C.; Yin, C. Co-delivery of doxorubicin and CRISPR/Cas9 or RNAi-expressing plasmid by chitosan-based nanoparticle for cancer therapy. Carbohydr. Polym. 2022, 287, 119315. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Samadi, F.Y.; Rahmani, S.; Mohammadi, Z. Chitosan-Raloxifene nanoparticle containing doxorubicin as a new double-effect targeting vehicle for breast cancer therapy. DARU J. Pharm. Sci. 2020, 28, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Das Kurmi, B.; Paliwal, R.; Paliwal, S.R. Dual cancer targeting using estrogen functionalized chitosan nanoparticles loaded with doxorubicin-estrone conjugate: A quality by design approach. Int. J. Biol. Macromol. 2020, 164, 2881–2894. [Google Scholar] [CrossRef] [PubMed]
- Karimifard, S.; Rezaei, N.; Jamshidifar, E.; Langeroodi, S.M.F.; Abdihaji, M.; Mansouri, A.; Hosseini, M.; Ahmadkhani, N.; Rahmati, Z.; Heydari, M.; et al. pH-Responsive Chitosan-Adorned Niosome Nanocarriers for Co-Delivery of Drugs for Breast Cancer Therapy. ACS Appl. Nano Mater. 2022, 5, 8811–8825. [Google Scholar] [CrossRef]
- Nivethaa, E.; Sankari, J.S.; Baskar, S.; Martin, C.A.; Sivanandham, N.; Kalkura, S.N. Enhanced inhibition of breast cancer by a dose reduced- dual anticancer drug loaded CMCS/Au nanocomposite. Mater. Lett. 2022, 318, 132123. [Google Scholar] [CrossRef]
- Lee, R.; Choi, Y.J.; Jeong, M.S.; Park, Y.I.; Motoyama, K.; Kim, M.W.; Kwon, S.-H.; Choi, J.H. Hyaluronic Acid-Decorated Glycol Chitosan Nanoparticles for pH-Sensitive Controlled Release of Doxorubicin and Celecoxib in Nonsmall Cell Lung Cancer. Bioconjugate Chem. 2020, 31, 923–932. [Google Scholar] [CrossRef]
- Chen, X.; Bremner, D.H.; Ye, Y.; Lou, J.; Niu, S.; Zhu, L.-M. A dual-prodrug nanoparticle based on chitosan oligosaccharide for enhanced tumor-targeted drug delivery. Colloids Surf. A 2021, 619, 126512. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, A.; Bharadwaj, R.; Ranganath, P.; Silverman, N.; Agrawal, G. Biodegradable disulfide crosslinked chitosan/stearic acid nanoparticles for dual drug delivery for colorectal cancer. Carbohydr. Polym. 2022, 294, 119833. [Google Scholar] [CrossRef]
- Upponi, J.R.; Jerajani, K.; Nagesha, D.K.; Kulkarni, P.; Sridhar, S.; Ferris, C.; Torchilin, V.P. Polymeric micelles: Theranostic co-delivery system for poorly water-soluble drugs and contrast agents. Biomaterials 2018, 170, 26–36. [Google Scholar] [CrossRef]
- Wu, W.; Chen, M.; Luo, T.; Fan, Y.; Zhang, J.; Zhang, Y.; Zhang, Q.; Sapin-Minet, A.; Gaucher, C.; Xia, X. ROS and GSH-responsive S-nitrosoglutathione functionalized polymeric nanoparticles to overcome multidrug resistance in cancer. Acta Biomater. 2020, 103, 259–271. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Tang, C.; Yin, C. Dual stimulus-responsive chitosan-based nanoparticles co-delivering doxorubicin and quercetin for cancer therapy. Mater. Lett. 2021, 305, 130826. [Google Scholar] [CrossRef]
- Chen, Q.; Jia, C.; Xu, Y.; Jiang, Z.; Hu, T.; Li, C.; Cheng, X. Dual-pH responsive chitosan nanoparticles for improving in vivo drugs delivery and chemoresistance in breast cancer. Carbohydr. Polym. 2022, 290, 119518. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Bai, J.; Zeng, Z.; Yang, X.; Wei, B.; Yang, Z. Cinnamaldehyde-modified chitosan hybrid nanoparticles for DOX delivering to produce synergistic anti-tumor effects. Front. Bioeng. Biotechnol. 2022, 10, 968065. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Z.; Fu, X.; Chen, Y.; Zhu, S.; Zhang, J. Co-delivery of Interleukin-12 and doxorubicin loaded Nano-delivery system for enhanced immunotherapy with polarization toward M1-type Macrophages. Eur. J. Pharm. Biopharm. 2022, 177, 175–183. [Google Scholar] [CrossRef]
- de Lima, J.M.; Castellano, L.R.C.; Bonan, P.R.F.; de Medeiros, E.S.; Hier, M.; Bijian, K.; Alaoui-Jamali, M.A.; da Cruz Perez, D.E.; da Silva, S.D. Chitosan/PCL nanoparticles can improve anti-neoplastic activity of 5-fluorouracil in head and neck cancer through autophagy activation. Int. J. Biochem. Cell Biol. 2021, 134, 105964. [Google Scholar] [CrossRef]
- Ullah, S.; Nawaz, A.; Farid, A.; Latif, M.S.; Fareed, M.; Ghazanfar, S.; Galanakis, C.M.; Alamri, A.S.; Alhomrani, M.; Asdaq, S.M.B. Folate-Modified Chitosan 5-Flourouraci Nanoparticles-Embedded Calcium Alginate Beads for Colon Targeted Delivery. Pharmaceutics 2022, 14, 1366. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Madni, A.; Shah, H.; Jan, N.; Shafiq, A.; Basit, A.; Rai, N.; Ali, A.; Khan, M.M. Folate decorated lipid chitosan hybrid nanoparticles of 5-fluorouracil for enhanced anticancer efficacy against colon cancer. Int. J. Biol. Macromol. 2022, 222, 497–508. [Google Scholar] [CrossRef]
- Cheng, M.; Dai, D. Inhibitory of active dual cancer targeting 5-Fluorouracil nanoparticles on liver cancer in vitro and in vivo. Front. Oncol. 2022, 12, 971475. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Wang, M.-H. Cerium oxide decorated 5-fluorouracil loaded chitosan nanoparticles for treatment of hepatocellular carcinoma. Int. J. Biol. Macromol. 2022, 216, 52–64. [Google Scholar] [CrossRef]
- Ajalli, N.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Díez-Pascual, A.M. Chitosan/Gamma-Alumina/Fe3O4@5-FU Nanostructures as Promising Nanocarriers: Physiochemical Characterization and Toxicity Activity. Molecules 2022, 27, 5369. [Google Scholar] [CrossRef]
- Wang, P.; Shen, Y.; Zhao, L. Chitosan nanoparticles loaded with aspirin and 5-fluororacil enable synergistic antitumour activity through the modulation of NF-κB/COX-2 signalling pathway. IET Nanobiotechnol. 2020, 14, 479–484. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Chen, G.C.; Qi, H.; Huang, K.; Hu, S. Preparation and synergistic chemo-photothermal therapy of redox-responsive carboxymethyl cellulose/chitosan complex nanoparticles. Carbohydr. Polym. 2022, 275, 118714. [Google Scholar] [CrossRef]
- Coutinho, A.J.; Lima, S.; Afonso, C.; Reis, S. Mucoadhesive and pH responsive fucoidan-chitosan nanoparticles for the oral delivery of methotrexate. Int. J. Biol. Macromol. 2020, 158, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Ai, Y.; Liu, F.; Chen, M.-M.; Liu, D. Lactobionic acid-modified thymine-chitosan nanoparticles as potential carriers for methotrexate delivery. Carbohydr. Res. 2021, 501, 108275. [Google Scholar] [CrossRef]
- Mazzotta, E.; De Benedittis, S.; Qualtieri, A.; Muzzalupo, R. Actively Targeted and Redox Responsive Delivery of Anticancer Drug by Chitosan Nanoparticles. Pharmaceutics 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.; Rani, S.; Kumar, V.; Nakhate, K.T.; Ajazuddin; Gupta, U. Sialic Acid Conjugated Chitosan Nanoparticles: Modulation to Target Tumour Cells and Therapeutic Opportunities. AAPS PharmSciTech 2022, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Elhabak, M.; Ibrahim, S.; Ibrahim, R.R. Intra-vaginal gemcitabine-hybrid nanoparticles for effective cervical cancer treatment. Opennano 2022, 8, 100090. [Google Scholar] [CrossRef]
- Geethakumari, D.; Sathyabhama, A.B.; Sathyan, K.R.; Mohandas, D.; Somasekharan, J.V.; Puthiyedathu, S.T. Folate functionalized chitosan nanoparticles as targeted delivery systems for improved anticancer efficiency of cytarabine in MCF-7 human breast cancer cell lines. Int. J. Biol. Macromol. 2022, 199, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Madni, A.; Filipczak, N.; Pan, J.; Rehman, M.; Rai, N.; Attia, S.A.; Torchilin, V.P. Folate targeted lipid chitosan hybrid nanoparticles for enhanced anti-tumor efficacy. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102228. [Google Scholar] [CrossRef]
- Khan, M.M.; Madni, A.; Tahir, N.; Parveen, F.; Khan, S.; Jan, N.; Ali, A.; Abdurrahim, M.; Farooq, U.; Khan, M.I. Co-Delivery of Curcumin and Cisplatin to Enhance Cytotoxicity of Cisplatin Using Lipid-Chitosan Hybrid Nanoparticles. Int. J. Nanomed. 2020, 15, 2207–2217. [Google Scholar] [CrossRef]
- Sultan, M.H.; Moni, S.S.; Madkhali, O.A.; Bakkari, M.A.; Alshahrani, S.; Alqahtani, S.S.; Alhakamy, N.A.; Mohan, S.; Ghazwani, M.; Bukhary, H.A.; et al. Characterization of cisplatin-loaded chitosan nanoparticles and rituximab-linked surfaces as target-specific injectable nano-formulations for combating cancer. Sci. Rep. 2022, 12, 468. [Google Scholar] [CrossRef]
- Matos, B.N.; Pereira, M.N.; Bravo, M.O.; Cunha-Filho, M.; Saldanha-Araújo, F.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles loading oxaliplatin as a mucoadhesive topical treatment of oral tumors: Iontophoresis further enhances drug delivery ex vivo. Int. J. Biol. Macromol. 2020, 154, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Bardal, S.K.; Waechter, J.E.; Martin, D.S. Neoplasia. In Applied Pharmacology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 305–324. ISBN 978-1-4377-0310-8. [Google Scholar]
- Wang, F.; Li, J.; Tang, X.; Huang, K.; Chen, L. Polyelectrolyte three layer nanoparticles of chitosan/dextran sulfate/chitosan for dual drug delivery. Colloids Surf. B 2020, 190, 110925. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Han, Y.; Xin, X.; Chen, L.; Liu, Y.; Liu, C.; Zhang, X.; Jin, M.; Jin, J.; Gao, Z.; et al. Biomimetic and temporal-controlled nanocarriers with ileum transporter targeting for achieving oral administration of chemotherapeutic drugs. J. Nanobiotechnol. 2022, 20, 281. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, Z.; Zheng, D.; Li, Z.; Zhao, Z. A targeted and redox/pH-responsive chitosan oligosaccharide derivatives based nanohybrids for overcoming multidrug resistance of breast cancer cells. Carbohydr. Polym. 2021, 251, 117008. [Google Scholar] [CrossRef]
- Mu, C.-F.; Cui, F.; Yin, Y.-M.; Cho, H.-J.; Kim, D.-D. Docetaxel-Loaded Chitosan-Cholesterol Conjugate-Based Self-Assembled Nanoparticles for Overcoming Multidrug Resistance in Cancer Cells. Pharmaceutics 2020, 12, 783. [Google Scholar] [CrossRef]
- Vikas; Viswanadh, M.K.; Mehata, A.K.; Sharma, V.; Priya, V.; Varshney, N.; Mahto, S.K.; Muthu, M.S. Bioadhesive chitosan nanoparticles: Dual targeting and pharmacokinetic aspects for advanced lung cancer treatment. Carbohydr. Polym. 2021, 274, 118617. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, Z.; Feng, L.; Deng, L.; Fang, Z.; Liu, Z.; Li, Y.; Wu, X.; Qin, L.; Guo, R.; et al. Chitosan-based nanoparticle co-delivery of docetaxel and curcumin ameliorates anti-tumor chemoimmunotherapy in lung cancer. Carbohydr. Polym. 2021, 268, 118237. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.D.; Øverbye, A.; Sahariah, P.; Gaware, V.S.; Høgset, H.; Másson, M.; Høgset, A.; Mælandsmo, G.M.; Skotland, T.; Sandvig, K.; et al. Drug-Loaded Photosensitizer-Chitosan Nanoparticles for Combinatorial Chemo- and Photodynamic-Therapy of Cancer. Biomacromolecules 2020, 21, 1489–1498. [Google Scholar] [CrossRef]
- Cannavà, C.; De Gaetano, F.; Stancanelli, R.; Venuti, V.; Paladini, G.; Caridi, F.; Ghica, C.; Crupi, V.; Majolino, D.; Ferlazzo, G.; et al. Chitosan-Hyaluronan Nanoparticles for Vinblastine Sulfate Delivery: Characterization and Internalization Studies on K-562 Cells. Pharmaceutics 2022, 14, 942. [Google Scholar] [CrossRef]
- Norman, A.W.; Litwack, G. Estrogens and Progestins. In Hormones; Elsevier: Amsterdam, The Netherlands, 1997; pp. 361–386. ISBN 978-0-12-521441-4. [Google Scholar]
- Waqas, M.K.; Safdar, S.; Buabeid, M.; Ashames, A.; Akhtar, M.; Murtaza, G. Alginate-coated chitosan nanoparticles for pH-dependent release of tamoxifen citrate. J. Exp. Nanosci. 2022, 17, 522–534. [Google Scholar] [CrossRef]
- Nokhodi, F.; Nekoei, M.; Goodarzi, M.T. Hyaluronic acid-coated chitosan nanoparticles as targeted-carrier of tamoxifen against MCF7 and TMX-resistant MCF7 cells. J. Mater. Sci. Mater. Med. 2022, 33, 24. [Google Scholar] [CrossRef] [PubMed]
- Alhajamee, M.; Marai, K.; Al Abbas, S.M.N.; Tabrizi, M.H. Co-encapsulation of curcumin and tamoxifen in lipid-chitosan hybrid nanoparticles for cancer therapy. Mater. Technol. 2022, 37, 1183–1194. [Google Scholar] [CrossRef]
- Yadav, A.S.; Radharani, N.N.V.; Gorain, M.; Bulbule, A.; Shetti, D.; Roy, G.; Baby, T.; Kundu, G.C. RGD functionalized chitosan nanoparticle mediated targeted delivery of raloxifene selectively suppresses angiogenesis and tumor growth in breast cancer. Nanoscale 2020, 12, 10664–10684. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, F.M.; El Rabey, H.A.; Tayel, A.A.; Alalawy, A.I.; Al-Duais, M.A.; Sakran, M.I.; Zidan, N.S. Augmented anticancer activity of curcumin loaded fungal chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 155, 861–867. [Google Scholar] [CrossRef]
- Valencia, M.S.; Júnior, M.F.D.S.; Xavier-Júnior, F.H.; Veras, B.D.O.; de Albuquerque, P.B.S.; Borba, E.F.D.O.; da Silva, T.G.; Xavier, V.L.; de Souza, M.P.; Carneiro-Da-Cunha, M.D.G. Characterization of curcumin-loaded lecithin-chitosan bioactive nanoparticles. Carbohydr. Polym. Technol. Appl. 2021, 2, 100119. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.G.; Jiao, W.-Q.; Yang, H.; Liu, J.; Liu, D. Phenylboronic acid-conjugated chitosan nanoparticles for high loading and efficient delivery of curcumin. Carbohydr. Polym. 2021, 256, 117497. [Google Scholar] [CrossRef]
- Alizadeh, N.; Malakzadeh, S. Antioxidant, antibacterial and anti-cancer activities of β-and γ-CDs/curcumin loaded in chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 147, 778–791. [Google Scholar] [CrossRef]
- Idoudi, S.; Hijji, Y.; Bedhiafi, T.; Korashy, H.M.; Uddin, S.; Merhi, M.; Dermime, S.; Billa, N. A novel approach of encapsulating curcumin and succinylated derivative in mannosylated-chitosan nanoparticles. Carbohydr. Polym. 2022, 297, 120034. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2021, 256, 117426. [Google Scholar] [CrossRef]
- Pazouki, N.; Irani, S.; Olov, N.; Atyabi, S.M.; Bagheri-Khoulenjani, S. Fe3O4 nanoparticles coated with carboxymethyl chitosan containing curcumin in combination with hyperthermia induced apoptosis in breast cancer cells. Prog. Biomater. 2022, 11, 43–54. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Khavani, M.; Bigham, A.; Sanati, A.; Bidram, E.; Shariati, L.; Zarrabi, A.; Jolfaie, N.A.; Rafienia, M. Mesoporous silica@chitosan@gold nanoparticles as “on/off” optical biosensor and pH-sensitive theranostic platform against cancer. Int. J. Biol. Macromol. 2022, 202, 241–255. [Google Scholar] [CrossRef]
- Dogan, M. Assessment of mechanism involved in the apoptotic and anti-cancer activity of Quercetin and Quercetin-loaded chitosan nanoparticles. Med. Oncol. 2022, 39, 176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Wang, S.; Gai, C.; Cui, X.; Xu, Z.; Li, W.; Zhang, W. Targeted delivery of quercetin by nanoparticles based on chitosan sensitizing paclitaxel-resistant lung cancer cells to paclitaxel. Mater. Sci. Eng. C 2021, 119, 111442. [Google Scholar] [CrossRef] [PubMed]
- Lazer, L.M.; Kesavan, Y.; Gor, R.; Ramachandran, I.; Pathak, S.; Narayan, S.; Anbalagan, M.; Ramalingam, S. Targeting colon cancer stem cells using novel doublecortin like kinase 1 antibody functionalized folic acid conjugated hesperetin encapsulated chitosan nanoparticles. Colloids Surf. B 2022, 217, 112612. [Google Scholar] [CrossRef] [PubMed]
- El-Houssiny, A.S.; Kamel, N.A.; Soliman, A.A.F.; El-Messieh, S.L.A.; Abd-El-Nour, K.N. Preparation and characterisation of gallic acid loaded carboxymethyl chitosan nanoparticles as drug delivery system for cancer treatment. Adv. Nat. Sci. Nanosci. Nanotechnol. 2022, 13, 025002. [Google Scholar] [CrossRef]
- Kaur, H.; Ghosh, S.; Kumar, P.; Basu, B.; Nagpal, K. Ellagic acid-loaded, tween 80-coated, chitosan nanoparticles as a promising therapeutic approach against breast cancer: In-vitro and in-vivo study. Life Sci. 2021, 284, 119927. [Google Scholar] [CrossRef]
- Samling, B.A.; Assim, Z.; Tong, W.-Y.; Leong, C.-R.; Ab Rashid, S.; Kamal, N.N.S.N.M.; Muhamad, M.; Tan, W.-N. Cynometra cauliflora essential oils loaded-chitosan nanoparticles: Evaluations of their antioxidant, antimicrobial and cytotoxic activities. Int. J. Biol. Macromol. 2022, 210, 742–751. [Google Scholar] [CrossRef]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Incorporation of Zataria multiflora essential oil into chitosan biopolymer nanoparticles: A nanoemulsion based delivery system to improve the in-vitro efficacy, stability and anticancer activity of ZEO against breast cancer cells. Int. J. Biol. Macromol. 2020, 143, 382–392. [Google Scholar] [CrossRef]
- Hesami, S.; Safi, S.; Larijani, K.; Badi, H.N.; Abdossi, V.; Hadidi, M. Synthesis and characterization of chitosan nanoparticles loaded with greater celandine (Chelidonium majus L.) essential oil as an anticancer agent on MCF-7 cell line. Int. J. Biol. Macromol. 2022, 194, 974–981. [Google Scholar] [CrossRef]
- Alipanah, H.; Farjam, M.; Zarenezhad, E.; Roozitalab, G.; Osanloo, M. Chitosan nanoparticles containing limonene and limonene-rich essential oils: Potential phytotherapy agents for the treatment of melanoma and breast cancers. BMC Complement. Med. Ther. 2021, 21, 186. [Google Scholar] [CrossRef]
- Valizadeh, A.; Khaleghi, A.A.; Alipanah, H.; Zarenezhad, E.; Osanloo, M. Anticarcinogenic Effect of Chitosan Nanoparticles Containing Syzygium aromaticum Essential Oil or Eugenol Toward Breast and Skin Cancer Cell Lines. Bionanoscience 2021, 11, 678–686. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Alipanah, H.; Baharifar, H.; Ranjbar, N.; Osanloo, M. Chitosan Nanoparticles Containing Cinnamomum verum J.Presl Essential Oil and Cinnamaldehyde; Preparation, Characterization and Anticancer Effects Against Melanoma and Breast Cancer Cells. Tradit. Integr. Med. 2022, 7, 1–12. [Google Scholar] [CrossRef]
- Rahmati, A.; Tabrizi, M.H.; Karimi, E.; Zarei, B. Fabrication and assessment of folic acid conjugated-chitosan modified PLGA nanoparticle for delivery of alpha terpineol in colon cancer. J. Biomater. Sci. Polym. Ed. 2022, 33, 1289–1307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Li, J.; Zhao, W.; Yang, Z.; Feng, Y. α-Santalol functionalized chitosan nanoparticles as efficient inhibitors of polo-like kinase in triple negative breast cancer. RSC Adv. 2020, 10, 5487–5501. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Gilani, S.J.; Bin Jumah, M.N.; Rizwanullah, M.; Zafar, A.; Ahmed, M.M.; Alshehri, S. Harnessing Lipid Polymer Hybrid Nanoparticles for Enhanced Oral Bioavailability of Thymoquinone: In Vitro and In Vivo Assessments. Polymers 2022, 14, 3705. [Google Scholar] [CrossRef]
- Ince, I.; Yıldırım, Y.; Güler, G.; Medine, E.I.; Ballıca, G.; Kuşdemir, B.C.; Göker, E. Synthesis and characterization of folic acid-chitosan nanoparticles loaded with thymoquinone to target ovarian cancer cells. J. Radioanal. Nucl. Chem. 2020, 324, 71–85. [Google Scholar] [CrossRef]
- Attallah, O.A.; Shetta, A.; Elshishiny, F.; Mamdouh, W. Essential oil loaded pectin/chitosan nanoparticles preparation and optimization via Box–Behnken design against MCF-7 breast cancer cell lines. RSC Adv. 2020, 10, 8703–8708. [Google Scholar] [CrossRef]
- Alirezaei, M.; Ghobeh, M.; Es-Haghi, A. Poly(lactic-co-glycolic acid)(PLGA)-based nanoparticles modified with chitosan-folic acid to delivery of Artemisia vulgaris L. essential oil to HT-29 cancer cells. Process. Biochem. 2022, 121, 207–215. [Google Scholar] [CrossRef]
- Tabrizi, M.H. Fabrication of folic acid-conjugated chitosan-coated PLGA nanoparticles for targeted delivery of Peganum harmala smoke extract to breast cancer cells. Nanotechnology 2022, 33, 495101. [Google Scholar] [CrossRef]
- Egil, A.C.; Ozdemir, B.; Gok, B.; Kecel-Gunduz, S.; Budama-Kilinc, Y. Synthesis, characterization, biological activities and molecular docking of Epilobium parviflorum aqueous extract loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 161, 947–957. [Google Scholar] [CrossRef]
- Khshemat, V.; Homayouni-Tabrizi, M.; Neamati, A.; Khadem, F.; Irani, M. Fabrication, Characterisation, and Biological Properties of Chitosan Nanoparticles Containing Rapeseed Pollen Extract (RPE) on the MCF-7 Cell Line. Mater. Technol. 2022, 37, 1075–1085. [Google Scholar] [CrossRef]










| Selected Terms * | 2020 | 2021 | 2022 |
|---|---|---|---|
| Chitosan nanoparticle(s), CS NP(s) | 1963 | 2159 | 2263 |
| CS NPs and drug delivery system(s)/targeted drug delivery | 503 | 504 | 522 |
| CS NP(s) AND cancer | 291 | 347 | 333 |
| CS NP(s) AND cisplatin | 14 | 22 | 16 |
| CS NP(s) AND doxorubicin | 87 | 87 | 73 |
| CS NP(s) AND methotrexate | 12 | 14 | 15 |
| CS NP(s) AND paclitaxel | 28 | 26 | 15 |
| CS NP(s) AND 5-fluorouracil | 23 | 19 | 26 |
| CS NP(s) AND curcumin | 72 | 80 | 86 |
| Type of Modification/Functionalization | Form of Loaded DOX | Carrier Advantages | Reference |
|---|---|---|---|
| Polyethylene glycol Monoclonal antibodies | Free | High selectivity High cytotoxicity against breast cancer cells | [29] |
| Polyethylene glycol High-intensity focused ultrasound | Free | Deep tumor penetration | [30] |
| Pluronic derivative | Free | Better drug concentration in tumor cells Higher oxidative stress | [34] |
| Oligosaccharides combined with benzaldehyde-terminated PEG | Imine bonds with benzaldehyde | pH sensitivity due to aniline bond destruction in the tumor environment | [36] |
| Magnetic core Folic acid | Free | External magnet support Targeted folate receptors | [38] |
| Catechol Hyaluronic acid | Free | High mucoadhesive ability Local drug administration | [39] |
| Benzylguanidine Lactobionic acid | Free | Targeted delivery to CXCR 4 positive tumors | [40] |
| Folic acid 2-(Diisopropyloamino) methacrylate | Free with co-loaded survivin | Combined gene- and chemotherapy Targeted folate receptors | [43] |
| Raloxifene | Free | combined therapy with selective estrogen receptor modulator | [44] |
| Estrone | Conjugate with estrone | Dual-targeted estrogen receptors | [45] |
| Glycol Hyaluronic acid | Attached to CS with co-loaded celecoxib | Synergism of active agents pH-sensitive drug attachment | [48] |
| Stearic acid | Free with co-loaded curcumin | Redox-sensitive disulfide bond Encapsulation of hydrophilic and hydrophobic drugs Synergism of active agents | [50] |
| Cinnamaldehyde Lactobionic acid | Free | Synergism of active agents Better antitumor effect Lower toxicity to healthy cells | [55] |
| Type of Modification/Functionalization | Loaded Drug | Carrier Advantages | Reference |
|---|---|---|---|
| Polycaprolactone | 5-FU | Higher antineoplastic activity in head and neck tumors | [57] |
| Folic acid Calcium alginate | 5-FU | Targeted folate receptors Stability in the gastric environment Mucoadhesive properties | [58] |
| Cerium oxide | 5-FU | The higher scavenging ability of reactive oxygen species | [61] |
| Polypyrrole Carboxymethyl cellulose | 5-FU | Synergistic chemo and photothermal therapy | [64] |
| Thymine Lactobionic acid | MTX | Improved drug uptake Improved targeting | [66] |
| L-cysteine Folic acid | MTX | Improved targeting | [67] |
| Lecithin | GEM | Improved vaginal penetration | [69] |
| Folic acid | CYT | Better targeting for folate-positive breast cancer cells | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurczewska, J. Chitosan-Based Nanoparticles with Optimized Parameters for Targeted Delivery of a Specific Anticancer Drug—A Comprehensive Review. Pharmaceutics 2023, 15, 503. https://doi.org/10.3390/pharmaceutics15020503
Kurczewska J. Chitosan-Based Nanoparticles with Optimized Parameters for Targeted Delivery of a Specific Anticancer Drug—A Comprehensive Review. Pharmaceutics. 2023; 15(2):503. https://doi.org/10.3390/pharmaceutics15020503
Chicago/Turabian StyleKurczewska, Joanna. 2023. "Chitosan-Based Nanoparticles with Optimized Parameters for Targeted Delivery of a Specific Anticancer Drug—A Comprehensive Review" Pharmaceutics 15, no. 2: 503. https://doi.org/10.3390/pharmaceutics15020503