In Vitro–In Vivo Correlations (IVIVC) for Predicting the Clinical Performance of Metronidazole Topical Creams Intended for Local Action
Abstract
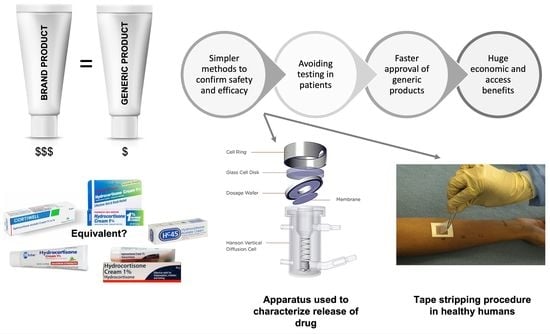
:1. Introduction
- Level A represents a point-to-point relationship between in vitro and in vivo profiles. Although these correlations are generally linear, non-linear correlations are also acceptable. It is considered to be the most informative and is the only level of IVIVC accepted by the FDA in obtaining biowaiver.
- Level B correlation utilizes the principles of statistical moment analysis. A mean in vitro dissolution time (MDTin vitro) is compared to either a mean in vivo residence (MRTin vivo) or dissolution time (MDTin vivo). However, since various in vivo release profiles may result in the same MRTin vivo or MDTin vivo, Level B correlation is not considered to be a point-to-point correlation. Additionally, this kind of correlation does not necessarily reflect the actual in vivo plasma profile, which may result in insufficient predictability.
- Level C involves a single-point correlation between a dissolution parameter (e.g., T50%, T90%) and a pharmacokinetic parameter such as peak plasma concentration (Cmax), time to reach Cmax (Tmax), or area under the curve (AUC). However, it may not be adequate in predicting in vivo drug performance because single-point analysis does not appropriately reflect the complete shape of the plasma concentration time-curve, which is critical to explain the in vivo performance of the formulation. Nevertheless, such correlations may be employed in the early stages of formulation development while selecting pilot formulations.
- Multiple Level C correlation relates multiple dissolution time points to one or more pharmacokinetic parameter(s) (e.g., Cmax, Tmax, or AUC). At least three dissolution time points covering the early, middle, and late stages of the dissolution profile should be included to establish such a correlation. A multiple Level C correlation can be as useful as a Level A correlation. Furthermore, the development of a multiple Level C correlation also indicates that establishing a more preferable Level A correlation is feasible.
Applications of IVIVC
2. Materials and Methods
2.1. Materials
2.2. Method
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US Food and Drug Administration. Guidance for Industry: Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations; US Food and Drug Administration: Silver Spring, MD, USA, 1997.
- Shen, J.; Burgess, D.J. In Vitro-In Vivo Correlation for Complex Non-Oral Drug Products: Where Do We Stand? J. Control. Release 2015, 219, 644–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, V.P. IV-IVC for Topically Applied Preparations—A Critical Evaluation. Eur. J. Pharm. Biopharm. 2005, 60, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Emami, J. In Vitro-In Vivo Correlation: From Theory to Applications. J. Pharm. Pharm. Sci. 2014, 9, 169–189. [Google Scholar]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Ibuprofen Transport into and through Skin from Topical Formulations: In Vitro-In Vivo Comparison. J. Investig. Dermatol. 2007, 127, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Caron, D.; Queille-Roussel, C.; Shah, V.P.; Schaefer, H. Correlation between the Drug Penetration and the Blanching Effect of Topically Applied Hydrocortisone Creams in Human Beings. J. Am. Acad. Dermatol. 1990, 23, 458–462. [Google Scholar] [CrossRef]
- Shah, V.P.; Elkins, J.; Skelly, J.P. Relationship between In Vivo Skin Blanching and In Vitro Release Rate for Betamethasone Valerate Creams. J. Pharm. Sci. 1992, 81, 104–106. [Google Scholar] [CrossRef]
- Cordery, S.F.; Pensado, A.; Chiu, W.S.; Shehab, M.Z.; Bunge, A.L.; Delgado-Charro, M.B.; Guy, R.H. Topical Bioavailability of Diclofenac from Locally-Acting, Dermatological Formulations. Int. J. Pharm. 2017, 529, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeheng, S.; Nosoongnoen, W.; Varothai, S.; Sathirakul, K. In Vitro-In Vivo Correlation Study for the Dermatopharmacokinetics of Terbinafine Hydrochloride Topical Cream. Drug Dev. Ind. Pharm. 2013, 39, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Welin-Berger, K.; Neelissen, J.A.M.; Emanuelsson, B.-M.; Björnsson, M.A.; Gjellan, K. In Vitro-In Vivo Correlation in Man of a Topically Applied Local Anesthetic Agent Using Numerical Convolution and Deconvolution. J. Pharm. Sci. 2003, 92, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In Vitro-In Vivo Correlation in Skin Permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Mateus, R.; Moore, D.J.; Hadgraft, J.; Lane, M.E. Percutaneous Absorption of Salicylic Acid—In Vitro and In Vivo Studies. Int. J. Pharm. 2014, 475, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Kanfer, I. A Validated IVRT Method to Assess Topical Creams Containing Metronidazole Using a Novel Approach. Pharmaceutics 2020, 12, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rath, S.; Ramanah, A.; Bon, C.; Kanfer, I. Application of a Dermatopharmacokinetic (DPK) Method for Bioequivalence Assessment of Topical Metronidazole Creams. J. Pharm. Pharm. Sci. 2020, 23, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, N.R.; Skinner, M.; Bon, C.; Kanfer, I. Bioequivalence of Topical Clotrimazole Formulations: An Improved Tape Stripping Method. J. Pharm. Pharm. Sci. 2011, 14, 347–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Au, W.L.; Skinner, M.; Kanfer, I. Comparison of Tape Stripping with the Human Skin Blanching Assay for the Bioequivalence Assessment of Topical Clobetasol Propionate Formulations. J. Pharm. Pharm. Sci. 2010, 13, 11–20. [Google Scholar] [CrossRef] [PubMed]


| Product | AUC (µg.% Skin Depth) | ARC (µg/cm2/min1/2) | Predicted AUC (µg.% Skin Depth) |
|---|---|---|---|
| 90% CI * (0.95–1.06) | |||
| Reference (run 1) | 77.46 ± 13.48 | 37.80 ± 2.01 | 78.40 |
| T1 (0.75% MTZ) | 77.62 ± 13.75 | 32.89 ± 1.60 | 71.62 |
| 90% CI * (0.74–0.84) | |||
| Reference (run 2) | 77.46 ± 13.48 | 38.47 ± 1.35 | 79.32 |
| T2 (0.56% MTZ) | 60.98 ± 10.30 | 27.42 ± 0.99 | 64.07 |
| 90% CI * (1.20–1.30) | |||
| Reference (run 2) | 77.46 ± 14.01 | 38.47 ± 1.35 | 79.32 |
| T3 (0.95% MTZ) | 96.78 ± 15.77 | 51.20 ± 0.60 | 96.89 |
| BE Limits | Predicted AUC (µg.% Skin Depth) | ARC (µg/cm2/min1/2) |
|---|---|---|
| 0.7999 (lower) | 68.33 | 30.50 |
| 1.2501 (upper) | 92.02 | 47.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rath, S.; Kanfer, I. In Vitro–In Vivo Correlations (IVIVC) for Predicting the Clinical Performance of Metronidazole Topical Creams Intended for Local Action. Pharmaceutics 2023, 15, 268. https://doi.org/10.3390/pharmaceutics15010268
Rath S, Kanfer I. In Vitro–In Vivo Correlations (IVIVC) for Predicting the Clinical Performance of Metronidazole Topical Creams Intended for Local Action. Pharmaceutics. 2023; 15(1):268. https://doi.org/10.3390/pharmaceutics15010268
Chicago/Turabian StyleRath, Seeprarani, and Isadore Kanfer. 2023. "In Vitro–In Vivo Correlations (IVIVC) for Predicting the Clinical Performance of Metronidazole Topical Creams Intended for Local Action" Pharmaceutics 15, no. 1: 268. https://doi.org/10.3390/pharmaceutics15010268