Development of a Solid Formulation Containing a Microemulsion of a Novel Artemisia Extract with Nematocidal Activity for Oral Administration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
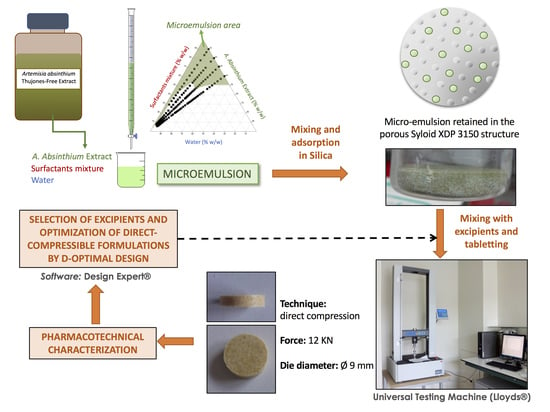
2.2.1. Formulation of a Microemulsion Containing the Artemisia absinthium Extract
2.2.2. Characterization of the Loaded Syloid® XDP System
2.2.3. Selection of the Binder, Binder Content and Compression Force
2.2.4. Optimization of a Tablet Formulation Based on a Syloid® XDP:ME System
3. Results
3.1. Characterization of the Loaded Syloid® XDP
3.2. Selection of the Binder, Binder Content and Compression Force
3.3. Development of Model Equations
3.3.1. Tensile Strength
3.3.2. Disintegration Time
3.4. Optimization of a Tablet Formulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moser, W.; Schindler, C.; Keiser, J. Efficacy of recommended drugs against soil transmitted helminths: Systematic review and network meta-analysis. BMJ 2017, 358, j4307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, S.; Gasser, R.B. Working towards new drugs against parasitic worms in a public-development partnership. Trends Parasitol. 2018, 34, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Beshay, E.V.N. Therapeutic efficacy of Artemisia absinthium against hymenolepis nana: In vitro and in vivo studies in comparison with the anthelmintic praziquantel. J. Helminthol. 2018, 92, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Perez-Roman, I.; Garcia-Rodriguez, J.; Kiekens, F.; Cordoba-Diaz, D.; Diaz, M. Enhanced nematocidal activity of a novel artemisia extract formulated as a microemulsion. Nat. Prod. Commun. 2019, 14, 1–6. [Google Scholar] [CrossRef]
- García-Rodríguez, J.J.; Andrés, M.F.; Ibañez-Escribano, A.; Julio, L.F.; Burillo, J.; Bolás-Fernández, F.; González-Coloma, A. Selective nematocidal effects of essential oils from two cultivated Artemisia absinthium populations. Z. Nat. C J. Biosci. 2015, 70, 275–280. [Google Scholar] [CrossRef]
- Hentzschel, C.M.; Sakmann, A.; Leopold, C.S. Suitability of various excipients as carrier and coating materials for liquisolid compacts. Drug Dev. Ind. Pharm. 2011, 37, 1200–1207. [Google Scholar] [CrossRef]
- Gumaste, S.G.; Pawlak, S.A.; Dalrymple, D.M.; Nider, C.J.; Trombetta, L.D.; Serajuddin, A.T.M. Development of solid SEDDS, IV: Effect of adsorbed lipid and surfactant on tableting properties and surface structures of different silicates. Pharm. Res. 2013, 30, 3170–3185. [Google Scholar] [CrossRef] [Green Version]
- Mandić, J.; Zvonar Pobirk, A.; Vrečer, F.; Gašperlin, M. Overview of solidification techniques for self-emulsifying drug delivery systems from industrial perspective. Int. J. Pharm. 2017, 533, 335–345. [Google Scholar] [CrossRef]
- Das, S.; Ng, W.K.; Tan, R.B.H. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef]
- Giannini, G.; Santaniello, M. A Versatile and stable mixture of fish oil and resveratrol in a powder formulation. Nutr. Food Technol. Open Access 2016, 2. [Google Scholar] [CrossRef]
- Tran, Q.T.; Vu, T.H.M.; Tran, V.T. Preparation of 200 mg fenofibrate hard capsule with high dissolution profile with microparticle entrapped micelles technology. Mahidol. Univ. J. Pharm. Sci. 2017, 44, 50–57. [Google Scholar] [CrossRef]
- Choudhari, Y.; Reddy, U.; Monsuur, F.; Pauly, T.; Hoefer, H.; McCarthy, W. Comparative evaluation of porous silica based carriers for lipids and liquid drug formulations. Mesoporous Biomater. 2014, 1, 61–74. [Google Scholar] [CrossRef]
- Lunter, D.J. Evaluation of mesoporous silica particles as drug carriers in hydrogels. Pharm. Dev. Technol. 2018, 23, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Madhav, K.V.; Kishan, V. Self microemulsifying particles of loratadine for improved oral bioavailability: Preparation, characterization and in vivo evaluation. J. Pharm. Investig. 2018, 48, 497–508. [Google Scholar] [CrossRef]
- Waters, L.J.; Hanrahan, J.P.; Tobin, J.M.; Finch, C.V.; Parkes, G.M.B.; Ahmad, S.A.; Mohammad, F.; Saleem, M. Enhancing the dissolution of phenylbutazone using Syloid® based mesoporous silicas for oral equine applications. J. Pharm. Anal. 2018, 8, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceutical on-line Journal—Application Note; Multifunctional Excipients for the Pharmaceutical Industry. Available online: https://www.pharmaceuticalonline.com/doc/multifunctional-excipients-for-the-pharmaceutical-industry-0001 (accessed on 13 September 2020).
- Chen, Y.; Wang, J.; Flanagan, D.R. Particle, powder and compact characterization. In Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice; Academic Press: Cambridge, MA, USA, 2016; pp. 271–296. [Google Scholar]
- Gumaste, S.G.; Dalrymple, D.M.; Serajuddin, A.T.M. Development of solid SEDDS, V: Compaction and drug release properties of tablets prepared by adsorbing lipid-based formulations onto Neusilin® US2. Pharm. Res. 2013, 30, 3186–3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinello, T.; Kaneko, T.M.; Velasco, M.V.R.; Taqueda, M.E.S.; Consiglieri, V.O. Optimization of poorly compactable drug tablets manufactured by direct compression using the mixture experimental design. Int. J. Pharm. 2006, 322, 87–95. [Google Scholar] [CrossRef]
- Swarbrick, J. Encyclopedia of Pharmaceutical Technology; Informa Healthcare: London, UK, 2007; ISBN 978-0-8493-9391-4. [Google Scholar]
- Rojas, J.J.; Aristizabal, J.; Henao, M. Screening of several excipients for direct compression of tablets: A new perspective based on functional properties. Rev. Ciênc. Farm. Básica E Appl. 2013, 34, 17–23. [Google Scholar]
- Mangal, S.; Meiser, F.; Morton, D.; Larson, I. Particle Engineering of excipients for direct compression: Understanding the role of material properties. Curr. Drug Metab. 2015, 21, 5877–5889. [Google Scholar] [CrossRef]
- Vialpando, M.; Aerts, A.; Persoons, J.; Martens, J.; Mooter, G.V.D. Evaluation of ordered mesoporous silica as a carrier for poorly soluble drugs: Influence of pressure on the structure and drug release. J. Pharm. Sci. 2011, 100, 3411–3420. [Google Scholar] [CrossRef]
- Mattsson, S.; Nyström, C. Evaluation of critical binder properties affecting the compactibility of binary mixtures. Drug Dev. Ind. Pharm. 2001, 27, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Tsai, M.-J.; Fang, Y.-P.; Fu, Y.-S.; Huang, Y.-B.; Wu, P.-C. Microemulsion formulation design and evaluation for hydrophobic compound: Catechin topical application. Colloids Surf. B Biointerfaces 2017, 161, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Ren, L.; Chen, G. Formulation optimization and ex vivo and in vivo evaluation of celecoxib microemulsion-based gel for transdermal delivery. AAPS PharmSciTech 2017, 18, 1960–1971. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, S.; Chaudhari, B. Optimization of microemulsion based transdermal gel of triamcinolone. Recent Patents Anti-Infect. Drug Disc. 2017, 12, 61–78. [Google Scholar] [CrossRef]
- Ansari, K.A.; Pagar, K.P.; Anwar, S.; Vavia, P.R.; Ansari, K.A.; Pagar, K.P.; Anwar, S.; Vavia, P.R. Design and optimization of self-microemulsifying drug delivery system (SMEDDS) of felodipine for chronotherapeutic application. Braz. J. Pharm. Sci. 2014, 50, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Dalvadi, H.; Patel, N.; Parmar, K. Systematic development of design of experiments (DoE) optimised self-microemulsifying drug delivery system of Zotepine. J. Microencapsul. 2017, 34, 308–318. [Google Scholar] [CrossRef]
- Valicherla, G.R.; Dave, K.M.; Syed, A.A.; Riyazuddin, M.; Gupta, A.P.; Singh, A.; Wahajuddin; Mitra, K.; Datta, D.; Gayen, J.R. Formulation optimization of Docetaxel loaded self-emulsifying drug delivery system to enhance bioavailability and anti-tumor activity. Sci. Rep. 2016, 6, 26895. [Google Scholar] [CrossRef]
- Ćurić, A.; Reul, R.; Möschwitzer, J.; Fricker, G. Formulation optimization of itraconazole loaded PEGylated liposomes for parenteral administration by using design of experiments. Int. J. Pharm. 2013, 448, 189–197. [Google Scholar] [CrossRef]
- Mura, P.; Capasso, G.; Maestrelli, F.; Furlanetto, S. Optimization of formulation variables of benzocaine liposomes using experimental design. J. Liposome Res. 2008, 18, 113–125. [Google Scholar] [CrossRef]
- Tibalinda, P.; Sempombe, J.; Shedafa, R.; Masota, N.; Pius, D.; Temu, M.; Kaale, E. Formulation development and optimization of Lamivudine 300 mg and Tenofovir Disoproxil Fumarate (TDF) 300 mg FDC tablets by D-optimal mixture design. Heliyon 2016, 2, e00207. [Google Scholar] [CrossRef] [Green Version]
- Yeom, D.W.; Chae, B.R.; Kim, J.H.; Chae, J.S.; Shin, D.J.; Kim, C.H.; Kim, S.R.; Choi, J.H.; Song, S.H.; Oh, D.; et al. Solid formulation of a supersaturable self-microemulsifying drug delivery system for valsartan with improved dissolution and bioavailability. Oncotarget 2017, 8, 94297–94316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamad Zen, N.I.; Abd Gani, S.S.; Shamsudin, R.; Fard Masoumi, H.R. The use of D-optimal mixture design in optimizing development of okara tablet formulation as a dietary supplement. Sci. World J. 2015, 2015, 684319. [Google Scholar] [CrossRef] [PubMed]
- Malakar, J.; Nayak, A.K.; Goswami, S. Use of response surface methodology in the formulation and optimization of bisoprolol fumarate matrix tablets for sustained drug release. ISRN Pharm. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolourtchian, N.; Hadidi, N.; Foroutan, S.M.; Shafaghi, B. Formulation and optimization of captopril sublingual tablet using D-optimal design. Iran. J. Pharm. Res. 2008, 7, 259–267. [Google Scholar]
- Maltais, M.; Vargas, R.; DiPaolo, T. Development of a new formulation for direct compression of a natural product. J. Pharm. Technol. Drug Res. 2015, 4, 2. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Wiley series in probability and statistics. In Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; Wiley: Hoboken, NJ, USA, 2016; ISBN 978-1-118-91601-8. [Google Scholar]
- He, S.; Ren, X.; Lu, Y.; Zhang, Y.; Wang, Y.; Sun, L. Microemulsification of clove essential oil improves its in vitro and in vivo control of Penicillium digitatum. Food Control. 2016, 65, 106–111. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Chow, A.H.L.; Ren, K.; Gong, T.; Zhang, Z.; Zheng, Y. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: Formulation and bioavailability studies. Int. J. Pharm. 2010, 383, 170–177. [Google Scholar] [CrossRef]
- United States Pharmacopeia and National Formulary (USP 38–NF 33); United States Pharmacopeial Convention: Rockville, MD, USA, 2016.
- Fell, J.T.; Newton, J.M. Determination of tablet strength by the diametral-compression test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef]
- Rowe, R.C. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009; ISBN 978-0-85369-792-3. [Google Scholar]
- Bühler, V. Soluble polyvinylpyrrolidone (Povidone). In Polyvinylpyrrolidone Excipients for Pharmaceuticals: Povidone, Crospovidone and Copovidone; Springer: Berlin, Germany, 2010; pp. 5–125. [Google Scholar]
- Said, M.; Elsayed, I.; Aboelwafa, A.A.; Elshafeey, A.H. Transdermal agomelatine microemulsion gel: Pyramidal screening, statistical optimization and in vivo bioavailability. Drug Deliv. 2017, 24, 1159–1169. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-B.; Tsai, Y.-H.; Yang, W.-C.; Chang, J.-S.; Wu, P.-C.; Takayama, K. Once-daily propranolol extended-release tablet dosage form: Formulation design and in vitro/in vivo investigation. Eur. J. Pharm. Biopharm. 2004, 58, 607–614. [Google Scholar] [CrossRef]
- Dean, A.; Morris, M.; Stufken, J.; Bingham, D. Handbook of Design and Analysis of Experiments; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Muzíková, J. Effect of magnesium stearate on the tensile strength of tablets made with the binder Prosolv SMCC 90. Ceska Slov. Farm. Cas. Ceske Farm. Spol. Slov. Farm. Spol. 2002, 51, 41–43. [Google Scholar]
- Jarosz, P.J.; Parrott, E.L. Effect of lubricants on tensile strengths of tablets. Drug Dev. Ind. Pharm. 1984, 10, 259–273. [Google Scholar] [CrossRef]
- Sheikh-salem, M.; Alkaysi, H.; Fell, J.T. The tensile strength of tablets of binary mixtures lubricated with magnesium stearate. Drug Dev. Ind. Pharm. 1988, 14, 895–903. [Google Scholar] [CrossRef]
- Muzíková, J.; Louzenska, M.; Pekarek, T. A study of compression process and properties of tablets with microcrystalline cellulose and colloidal silicon dioxide. Acta Poliniae Pharm. 2016, 73, 1259–1265. [Google Scholar]
- Pingali, K.; Mendez, R.; Lewis, D.; Michniak-Kohn, B.; Cuitino, A.; Muzzio, F. Mixing order of glidant and lubricant—Influence on powder and tablet properties. Int. J. Pharm. 2011, 409, 269–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, V.; Siddiqui, A.; Ali, H.; Nazzal, S. Dissolution and powder flow characterization of solid self-emulsified drug delivery system (SEDDS). Int. J. Pharm. 2009, 366, 44–52. [Google Scholar] [CrossRef]
- Chen, L.; Ding, X.; He, Z.; Huang, Z.; Kunnath, K.T.; Zheng, K.; Davé, R.N. Surface engineered excipients: I. improved functional properties of fine grade microcrystalline cellulose. Int. J. Pharm. 2018, 536, 127–137. [Google Scholar] [CrossRef]
- Ferrero, C.; Muñoz, N.; Velasco, M.V.; Muñoz-Ruiz, A.; Jiménez-Castellanos, R. Disintegrating efficiency of croscarmellose sodium in a direct compression formulation. Int. J. Pharm. 1997, 147, 11–21. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Wang, Y.; Liu, X.; Liu, Y.; Fu, Q.; Meng, P.; He, Z. Solid self-emulsifying nitrendipine pellets: Preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2010, 383, 1–6. [Google Scholar] [CrossRef]
- Mirani, A.G.; Patankar, S.P.; Borole, V.S.; Pawar, A.S.; Kadam, V.J. Direct compression high functionality excipient using coprocessing technique: A brief review. Curr. Drug Deliv. 2011, 8, 426–435. [Google Scholar] [CrossRef]
- Sun, C.C.; Hou, H.; Gao, P.; Ma, C.; Medina, C.; Alvarez, F.J.; Hou, H.; Gao, P. Development of a high drug load tablet formulation based on assessment of powder manufacturability: Moving towards quality by design. J. Pharm. Sci. 2009, 98, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kachrimanis, K.; Nikolakakis, I.; Malamataris, S. Tensile strength and disintegration of tableted silicified microcrystalline cellulose: Influences of interparticle bonding. J. Pharm. Sci. 2003, 92, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Tye, C.K.; Sun, C.C.; Amidon, G.E. Evaluation of the effects of tableting speed on the relationships between compaction pressure, tablet tensile strength, and tablet solid fraction. J. Pharm. Sci. 2005, 94, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Diarra, H.; Mazel, V.; Busignies, V.; Tchoreloff, P. Investigating the effect of tablet thickness and punch curvature on density distribution using finite elements method. Int. J. Pharm. 2015, 493, 121–128. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Revision of Monograph on Tablets. Final Text for Addition to The International Pharmacopoeia; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Al-Gousous, J.; Langguth, P. Oral solid dosage form disintegration testing—The forgotten test. J. Pharm. Sci. 2015, 104, 2664–2675. [Google Scholar] [CrossRef] [Green Version]



| Component | Minimum (% w/w) | Maximum (% w/w) |
|---|---|---|
| Syloid® XDP:ME (1:1.5) | 32 | 60 |
| MCC | 20 | 40 |
| PVP | 0 | 20 |
| CCMNa | 0 | 6 |
| Syloid® 244 | 0 | 1 |
| MgSt | 0.5 | 1 |
| Parameter | Syloid® XDP | Syloid® XDP:ME (1:1.5) |
|---|---|---|
| Bulk density (g/mL) | 0.281 ± 0.000 | 0.730 ± 0.029 |
| Tapped density (g/mL) | 0.319 ± 0.000 | 0.882 ± 0.028 |
| Compressibility index | 12.000 ± 0.000 | 17.216 ± 3.291 |
| Hausner ratio | 1.136 ± 0.000 | 1.210 ± 0.049 |
| Angle of repose | 26.589 ± 1.142 | 21.608 ± 2.636 |
| Exp | Syloid® XDP:ME (1:1.5) (% w/w) | MCC (% w/w) | PVP (% w/w) | CCMNa (% w/w) | Syloid® 244 (% w/w) | MgSt (% w/w) |
|---|---|---|---|---|---|---|
| 1 | 42.89 | 29.76 | 19.41 | 5.94 | 1.00 | 1.00 |
| 2 | 56.53 | 40.00 | 0.00 | 2.19 | 0.73 | 0.56 |
| 3 | 52.23 | 23.09 | 20.00 | 3.41 | 0.66 | 0.62 |
| 4 | 42.69 | 39.90 | 15.67 | 0.00 | 0.75 | 0.99 |
| 5 | 52.23 | 23.09 | 20.00 | 3.41 | 0.66 | 0.62 |
| 6 | 36.16 | 39.67 | 16.69 | 6.00 | 0.66 | 0.82 |
| 7 | 46.99 | 40.00 | 5.10 | 6.00 | 0.91 | 1.00 |
| 8 | 53.92 | 29.60 | 14.49 | 0.00 | 0.99 | 1.00 |
| 9 | 55.55 | 30.94 | 5.52 | 6.00 | 1.00 | 1.00 |
| 10 | 60.00 | 24.03 | 11.74 | 3.29 | 0.08 | 0.86 |
| 11 | 48.58 | 35.89 | 11.23 | 3.03 | 0.52 | 0.75 |
| 12 | 60.00 | 24.03 | 11.74 | 3.30 | 0.08 | 0.86 |
| 13 | 48.25 | 30.29 | 16.10 | 3.38 | 0.99 | 0.99 |
| 14 | 42.69 | 39.90 | 15.67 | 0.00 | 0.75 | 0.99 |
| 15 | 56.52 | 40.00 | 0.00 | 2.19 | 0.73 | 0.56 |
| 16 | 36.16 | 39.67 | 16.70 | 6.00 | 0.66 | 0.82 |
| Exp. | Bulk Density | Tapped Density | Comp. Index | Hausner Ratio | Flow *,† | Angle of Repose | Flow *,‡ |
|---|---|---|---|---|---|---|---|
| 1 | 0.44 ± 0.00 | 0.48 ± 0.00 | 9.78 ± 0.00 | 1.11 ± 0.00 | Excellent | 16 ± 2 | Excellent |
| 2 | 0.51 ± 0.00 | 0.57 ± 0.00 | 11.11 ± 0.00 | 1.13 ± 0.00 | Good | 20 ± 1 | Excellent |
| 3 | 0.42 ± 0.01 | 0.50 ± 0.02 | 15.81 ± 2.98 | 1.19 ± 0.04 | Fair | 23 ± 3 | Excellent |
| 4 | 0.39 ± 0.02 | 0.44 ± 0.01 | 11.00 ± 2.80 | 1.12 ± 0.04 | Good | 16 ± 1 | Excellent |
| 5 | 0.42 ± 0.01 | 0.44 ± 0.14 | 5.50 ± 2.98 | 1.06 ± 0.03 | Excellent | 16 ± 6 | Excellent |
| 6 | 0.36 ± 0.00 | 0.45 ± 0.00 | 21.05 ± 0.00 | 1.28 ± 0.00 | Passable | 24 ± 3 | Excellent |
| 7 | 0.45 ± 0.00 | 0.50 ± 0.00 | 11.11 ± 0.00 | 1.13 ± 0.00 | Good | 7 ± 4 | Excellent |
| 8 | 0.33 ± 0.00 | 0.40 ± 0.00 | 16.67 ± 0.00 | 1.20 ± 0.00 | Fair | 12 ± 5 | Excellent |
| 9 | 0.40 ± 0.00 | 0.47 ± 0.00 | 15.00 ± 0.00 | 1.18 ± 0.00 | Good | 25 ± 6 | Excellent |
| 10 | 0.37 ± 0.00 | 0.45 ± 0.00 | 18.18 ± 0.00 | 1.22 ± 0.00 | Fair | 24 ± 1 | Excellent |
| 11 | 0.41 ± 0.00 | 0.45 ± 0.00 | 7.69 ± 0.00 | 1.08 ± 0.00 | Excellent | 25 ± 2 | Excellent |
| 12 | 0.42 ± 0.00 | 0.50 ± 0.00 | 16.60 ± 0.00 | 1.20 ± 0.00 | Fair | 24 ± 2 | Excellent |
| 13 | 0.42 ± 0.00 | 0.48 ± 0.02 | 12.28 ± 3.04 | 1.14 ± 0.04 | Good | 22 ± 1 | Excellent |
| 14 | 0.30 ± 0.00 | 0.38 ± 0.00 | 20.46 ± 0.00 | 1.26 ± 0.00 | Passable | 25 ± 3 | Excellent |
| 15 | 0.50 ± 0.00 | 0.57 ± 0.00 | 12.50 ± 0.00 | 1.14 ± 0.00 | Good | 19 ± 0 | Excellent |
| 16 | 0.31 ± 0.00 | 0.35 ± 0.00 | 11.54 ± 0.00 | 1.13 ± 0.00 | Good | 21 ± 2 | Excellent |
| Experiment | Tensile Strength (MPa) | Disintegration Time (min) | ||
|---|---|---|---|---|
| Minimum Value | Maximum Value | Average ± SD | ||
| 1 | 1.821 | 2.291 | 2.052 ± 0.194 | 5.600 |
| 2 | 0.358 | 0.420 | 0.388 ± 0.023 | 0.300 |
| 3 | 2.257 | 3.166 | 2.703 ± 0.401 | 5.550 |
| 4 | 2.084 | 2.379 | 2.230 ± 0.103 | 14.000 |
| 5 | 2.024 | 3.016 | 2.510 ± 0.446 | 9.217 |
| 6 | 2.570 | 2.736 | 2.652 ± 0.030 | 6.400 |
| 7 | 0.794 | 0.977 | 0.884 ± 0.074 | 1.500 |
| 8 | 1.908 | 2.497 | 2.197 ± 0.251 | 9.417 |
| 9 | 0.000 | 0.000 | 0.000 ± 0.000 | 0.000 |
| 10 | 2.361 | 3.045 | 2.697 ± 0.288 | 5.667 |
| 11 | 0.776 | 0.779 | 0.777 ± 0.000 | 0.000 |
| 12 | 0.133 | 0.137 | 0.136 ± 0.000 | 5.776 |
| 13 | 1.953 | 3.205 | 2.567 ± 0.574 | 7.383 |
| 14 | 2.098 | 2.487 | 2.289 ± 0.149 | 0.383 |
| 15 | 0.412 | 0.630 | 0.519 ± 0.099 | 0.200 |
| 16 | 2.263 | 2.956 | 2.603 ± 0.295 | 6.958 |
| Parameter | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Tensile Strength (MPa) | |||||
| Model | 8.43 | 5 | 1.69 | 19.58 | 0.0001 |
| Linear mixture | 8.43 | 5 | 1.69 | 19.58 | 0.0001 |
| Residual | 0.78 | 9 | 0.09 | - | - |
| Lack of fit | 0.75 | 5 | 0.15 | 19.86 | 0.0063 |
| Pure error | 0.03 | 4 | 0.01 | - | - |
| Corrected Sum of Squares | 9.21 | 14 | - | - | - |
| Disintegration Time (min) | |||||
| Model | 85.09 | 5 | 17.02 | 12.06 | 0.0044 |
| Linear mixture | 85.09 | 5 | 17.02 | 12.06 | 0.0044 |
| Residual | 8.47 | 6 | 1.41 | - | - |
| Lack of fit | 1.59 | 3 | 0.53 | 0.230 | 0.8700 |
| Pure error | 6.88 | 3 | 2.29 | - | - |
| Corrected Sum of Squares | 93.56 | 11 | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Roman, I.; Kiekens, F.; Cordoba-Diaz, D.; Garcia-Rodriguez, J.J.; Cordoba-Diaz, M. Development of a Solid Formulation Containing a Microemulsion of a Novel Artemisia Extract with Nematocidal Activity for Oral Administration. Pharmaceutics 2020, 12, 873. https://doi.org/10.3390/pharmaceutics12090873
Perez-Roman I, Kiekens F, Cordoba-Diaz D, Garcia-Rodriguez JJ, Cordoba-Diaz M. Development of a Solid Formulation Containing a Microemulsion of a Novel Artemisia Extract with Nematocidal Activity for Oral Administration. Pharmaceutics. 2020; 12(9):873. https://doi.org/10.3390/pharmaceutics12090873
Chicago/Turabian StylePerez-Roman, Ines, Filip Kiekens, Damian Cordoba-Diaz, Juan Jose Garcia-Rodriguez, and Manuel Cordoba-Diaz. 2020. "Development of a Solid Formulation Containing a Microemulsion of a Novel Artemisia Extract with Nematocidal Activity for Oral Administration" Pharmaceutics 12, no. 9: 873. https://doi.org/10.3390/pharmaceutics12090873