The Effect of Compression Pressure on the First Layer Surface Roughness and Delamination of Metformin and Evogliptin Bilayer and Trilayer Tablets
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological and Physical Characteristics of MF and EG Granules
2.2. Effect of Compression Pressure on the Porosity and Compaction Breaking Force of MF/EG MLTs
2.3. Effect of PRE-P on the Interfacial Strength of MF/EG MLTs
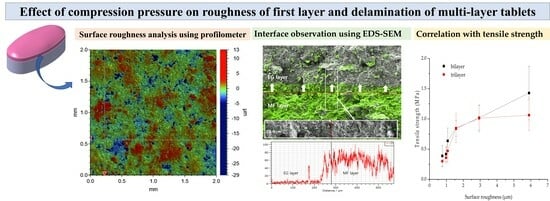
2.4. Effect of Pre-Compression Pressure on the Surface Roughness of MF/EG Bilayer Tablets
2.5. EDS-Equipped SEM Observation of MF/EG Bilayer Tablets
3. Materials and Methods
3.1. Materials
3.2. Preparation of MF and EG Granules Using the Wet Granulation Method
3.3. Characterization of MF and EG Granules Prepared by the Wet Granulation Method
3.4. Preparation of MF/EG-Loaded Bilayer and Trilayer Tablets
3.5. Porosity of MF/EG-Loaded MLTs Depending on Compression Pressure
3.6. Compaction force Required to Break MF/EG-Loaded MLTs
3.7. Interfacial Strength of MF/EG-Loaded MLTs
3.8. Determination of Surface Topography of MF/EG-Loaded MLTs
3.9. EDS-Equipped SEM Observations of Interfaces of Bilayer Tablets
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deshpande, R.D.; Gowda, D.V.; Mahammed, N.; Maramwar, D.N. Bi-layer tablets-An emerging trend: A review. Int. J. Pharm. Sci. Res. 2011, 2, 2534. [Google Scholar] [CrossRef]
- Abdul, S.; Poddar, S.S. A flexible technology for modified release of drugs: Multi layered tablets. J. Control. Release 2004, 97, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Rameshwar, V.; Kishor, D.; Tushar, G. Bi-layer tablets for various drugs: A review. Sch. Acad. J. Pharm. 2014, 3, 271–279. [Google Scholar]
- He, W.; Li, Y.; Zhang, R.; Wu, Z.; Yin, L. Gastro-floating bilayer tablets for the sustained release of metformin and immediate release of pioglitazone: Preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2014, 476, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.N.; Kumar, A.B.; Pandit, H.K.; Singh, S.P.; Devi, M.V. Design and evaluation of sustained release bilayer tablets of propranolol hydrochloride. Acta Pharm. 2007, 57, 479–489. [Google Scholar] [CrossRef]
- Mandal, U.; Pal, T.K. Formulation and in vitro studies of a fixed-dose combination of a bilayer matrix tablet containing metformin HCl as sustained release and glipizide as immediate release. Drug Dev. Ind. Pharm. 2008, 34, 305–313. [Google Scholar] [CrossRef]
- Simão, J.; Chaudhary, S.A.; Ribeiro, A.J. Implementation of quality by design (QbD) for development of bilayer tablets. Eur. J. Pharm. Sci. 2023, 184, 106412. [Google Scholar] [CrossRef]
- Akseli, I.; Abebe, A.; Sprockel, O.; Cuitiño, A.M. Mechanistic characterization of bilayer tablet formulations. Powder Technol. 2013, 236, 30–36. [Google Scholar] [CrossRef]
- Inman, S.J.; Briscoe, B.J.; Pitt, K.G. Determination of the Critical Stress Intensity Factor (KIC) for Microcrystalline Cellulose (MCC) Using a Developed Tensile Stress Method and Its Application to Double-Layer Tablets; American Institute of Chemical Engineers: New York, NY, USA, 2006. [Google Scholar]
- Niwa, M.; Hiraishi, Y.; Iwasaki, N.; Terada, K. Quantitative analysis of the layer separation risk in bilayer tablets using terahertz pulsed imaging. Int. J. Pharm. 2013, 452, 249–256. [Google Scholar] [CrossRef]
- Akseli, I.; Dey, D.; Cetinkaya, C. Mechanical property characterization of bilayered tablets using nondestructive air-coupled acoustics. AAPS PharmSciTech 2010, 11, 90–102. [Google Scholar] [CrossRef]
- Kottala, N.; Abebe, A.; Sprockel, O.; Bergum, J.; Nikfar, F.; Cuitiño, A.M. Evaluation of the performance characteristics of bilayer tablets: Part I. Impact of material properties and process parameters on the strength of bilayer tablets. AAPS PharmSciTech 2012, 13, 1236–1242. [Google Scholar] [CrossRef]
- Abebe, A.; Akseli, I.; Sprockel, O.; Kottala, N.; Cuitiño, A.M. Review of bilayer tablet technology. Int. J. Pharm. 2014, 461, 549–558. [Google Scholar] [CrossRef]
- Chang, S.Y.; Sun, C.C. Superior plasticity and tabletability of theophylline monohydrate. Mol. Pharm. 2017, 14, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Sun, C.C. Interfacial bonding in formulated bilayer tablets. Eur. J. Pharm. Biopharm. 2020, 147, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Karehill, P.G.; Glazer, M.; Nyström, C. Studies on direct compression of tablets. XXIII. The importance of surface roughness for the compactability of some directly compressible materials with different bonding and volume reduction properties. Int. J. Pharm. 1990, 64, 35–43. [Google Scholar] [CrossRef]
- Kottala, N.; Abebe, A.; Sprockel, O.; Akseli, I.; Nikfar, F.; Cuitiño, A.M. Influence of compaction properties and interfacial topography on the performance of bilayer tablets. Int. J. Pharm. 2012, 436, 171–178. [Google Scholar] [CrossRef]
- Zou, P.; Guo, M.; Hu, J. Evogliptin for the treatment option for type 2 diabetes: An update of the literature. Expert. Rev. Clin. Pharmacol. 2022, 15, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Jeong, H.E.; Oh, I.S.; Hong, S.M.; Yu, S.H.; Lee, C.B.; Shin, J.Y. Cardiovascular safety of evogliptin in patients with type 2 diabetes: A nationwide cohort study. Diabetes Obes. Metab. 2021, 23, 1232–1241. [Google Scholar] [CrossRef]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Chacra, A.R. Evolving metformin treatment strategies in type-2 diabetes: From immediate-release metformin monotherapy to extended-release combination therapy. Am. J. Ther. 2014, 21, 198–210. [Google Scholar] [CrossRef]
- Jabbour, S.; Ziring, B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgrad. Med. 2011, 123, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.D.; Liu, Y.; Tang, X.; Zhang, Q. Preparation and in vitro/in vivo evaluation of sustained-release metformin hydrochloride pellets. Eur. J. Pharm. Biopharm. 2006, 64, 185–192. [Google Scholar] [CrossRef] [PubMed]
- USP Standard, No. 1174; Powder Flow. The United States Pharmacopeial Convention: North Bethesda, MD, USA, 2019. Available online: https://www.usp.org/harmonization-standards/pdg/general-chapters/powder-flow (accessed on 23 October 2023).
- Saritha, G.; Iswarya, T.; Keerthana, D.; Baig, A.T.D. Micro universal testing machine system for material property measurement. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Chang, S.Y.; Li, J.X.; Sun, C.C. Tensile and shear methods for measuring strength of bilayer tablets. Int. J. Pharm. 2017, 523, 121–126. [Google Scholar] [CrossRef]
- Podczeck, F.; Newton, J.M.; Fromme, P. The bending strength of tablets with a breaking line—Comparison of the results of an elastic and a “brittle cracking” finite element model with experimental findings. Int. J. Pharm. 2015, 495, 485–499. [Google Scholar] [CrossRef]
- Shang, C.; Sinka, I.C.; Pan, J. Modelling of the break force of tablets under diametrical compression. Int. J. Pharm. 2013, 445, 99–107. [Google Scholar] [CrossRef]
- Busignies, V.; Mazel, V.; Diarra, H.; Tchoreloff, P. Development of a new test for the easy characterization of the adhesion at the interface of bilayer tablets: Proof-of-concept study by experimental design. Int. J. Pharm. 2014, 477, 476–484. [Google Scholar] [CrossRef]
- Vaithiyalingam, S.R.; Sayeed, V.A. Critical factors in manufacturing multi-layer tablets—Assessing material attributes, in-process controls, manufacturing process and product performance. Int. J. Pharm. 2010, 398, 9–13. [Google Scholar] [CrossRef]
- Kottala, N.; Abebe, A.; Sprockel, O.; Akseli, I.; Nikfar, F.; Cuitiño, A.M. Characterization of interfacial strength of layered powder-compacted solids. Powder Technol. 2013, 239, 300–307. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.Y.; Storey, D.; Byrne, G. Interfacial strength of bilayer pharmaceutical tablets. Powder Technol. 2018, 337, 36–42. [Google Scholar] [CrossRef]
- Ohsaki, S.; Kushida, K.; Matsuda, Y.; Nakamura, H.; Watano, S. Numerical study for tableting process in consideration of compression speed. Int. J. Pharm. 2020, 575, 118936. [Google Scholar] [CrossRef]
- Sugimori, K.; Mori, S.; Kawashima, Y. Characterization of die wall pressure to predict capping of flat-or convex-faced drug tablets of various sizes. Powder Technol. 1989, 58, 259–264. [Google Scholar] [CrossRef]
- Akseli, I.; Hancock, B.C.; Cetinkaya, C. Non-destructive determination of anisotropic mechanical properties of pharmaceutical solid dosage forms. Int. J. Pharm. 2009, 377, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Long, W.M.; Alderton, J.R. The displacement of gas from powders during compaction. Powder Metall. 1960, 3, 52–72. [Google Scholar] [CrossRef]
- Lee, D.H.; Cho, N.G. Assessment of surface profile data acquired by a stylus profilometer. Meas. Sci. Technol. 2012, 23, 105601. [Google Scholar] [CrossRef]
- Verran, J.; Boyd, R.D. The relationship between substratum surface roughness and microbiological and organic soiling: A review. Biofouling 2001, 17, 59–71. [Google Scholar] [CrossRef]
- Chang, S.Y.; Sun, C.C. Insights into the effect of compaction pressure and material properties on interfacial bonding strength of bilayer tablets. Powder Technol. 2019, 354, 867–876. [Google Scholar] [CrossRef]
- Inman, S.J.; Briscoe, B.J.; Pitt, K.G. Topographic characterization of cellulose bilayered tablets interfaces. Chem. Eng. Res. Des. 2007, 85, 1005–1012. [Google Scholar] [CrossRef]
- Desai, D.; Wang, J.; Wen, H.; Li, X.; Timmins, P. Formulation design, challenges, and development considerations for fixed dose combination (FDC) of oral solid dosage forms. Pharm. Dev. Technol. 2013, 18, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Won, D.H.; Park, H.; Ha, E.S.; Kim, H.H.; Jang, S.W.; Kim, M.S. Optimization of bilayer tablet manufacturing process for fixed dose combination of sustained release high-dose drug and immediate release low-dose drug based on quality by design (QbD). Int. J. Pharm. 2021, 605, 120838. [Google Scholar] [CrossRef]
- Razvi SZ, A.; Kamm, I.; Nguyen, T.; Pellett, J.D.; Kumar, A. Loss on drying using halogen moisture analyzer: An orthogonal technique for monitoring volatile content for in-process control samples during pharmaceutical manufacturing. Org. Process Res. Dev. 2021, 25, 300–307. [Google Scholar] [CrossRef]
- Ouyang, H.; Zheng, A.Y.; Heng, P.W.S.; Chan, L.W. Effect of lipid additives and drug on the rheological properties of molten paraffin wax, degree of surface drug coating, and drug release in spray-congealed microparticles. Pharmaceutics 2018, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- USP Standard, No. 616; Bulk Density and Tapped Density. The United States Pharmacopeial Convention: Rockville, MD, USA, 2015. Available online: https://www.usp.org/sites/default/files/usp/document/harmonization/gen-chapter/bulk_density.pdf (accessed on 23 October 2023).
- ISO Standard, No. 4287; Geometrical Product Specification (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 1997. Available online: https://www.iso.org/standard/10132.html (accessed on 23 October 2023).







| Function | Ingredient | Content (mg) | ||
|---|---|---|---|---|
| Bilayer Tablet | Trilayer Tablet | |||
| Third Layer | Active substance | MF | - | 500 |
| Binder | PVP K30 | 15 | ||
| Lubricant | Magnesium stearate | 2.5 | ||
| Controlled release excipient | Carbomer 934P | 17.5 | ||
| Controlled release excipient | HPMC2208 | 65 | ||
| Controlled release excipient | Methacrylate copolymer | 30 | ||
| Second layer | Active substance | EG | 6.8 | 6.8 |
| Diluent | Pregelatinized starch | 9.0 | 9.0 | |
| Diluent | Mannitol | 71.1 | 71.1 | |
| Disintegrant | Ac-Di-Sol | 13.5 | 13.5 | |
| Disintegrant | L-HPC | 9 | 9 | |
| Glidant | Colloidal silicon dioxide | 1.3 | 1.3 | |
| Lubricant | Magnesium stearate | 3.1 | 3.1 | |
| Colorant | Iron oxide | 0.3 | 0.3 | |
| Binder | HPC | 2.7 | 2.7 | |
| First layer | Active substance | MF | 1000 | 500 |
| Binder | PVP K30 | 30 | 15 | |
| Lubricant | Magnesium stearate | 5.0 | 2.5 | |
| Controlled release excipient | Carbomer 934P | 35 | 17.5 | |
| Controlled release excipient | HPMC2208 | 130 | 65 | |
| Controlled release excipient | Methacrylate copolymer | 60 | 30 | |
| EG Granules | MF Granules | |
|---|---|---|
| LOD (%) 1 | 0.70 ± 0.09 | 0.74 ± 0.09 |
| BD (g/mL) 1 | 0.46 ± 0.01 | 0.43 ± 0.01 |
| TD (g/mL) 1 | 0.51 ± 0.01 | 0.48 ± 0.01 |
| HR 1,2 | 1.11 ± 0.02 | 1.12 ± 0.02 |
| CI (%) 1,3 | 9.79 ± 1.89 | 10.48 ± 1.90 |
| Particle size d0.1 (μm) 1,4 | 16.18 ± 0.22 | 10.73 ± 0.25 |
| Particle size d0.5 (μm) 1,5 | 38.33 ±1.86 | 44.74 ± 2.19 |
| Particle size d0.9 (μm) 1,6 | 83.75 ± 2.74 | 110.93 ± 4.23 |
| SPAN 1,7 | 1.76 ± 0.02 | 2.24 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Kook, J.H.; Seo, D.-W.; Kang, M.J. The Effect of Compression Pressure on the First Layer Surface Roughness and Delamination of Metformin and Evogliptin Bilayer and Trilayer Tablets. Pharmaceuticals 2023, 16, 1523. https://doi.org/10.3390/ph16111523
Kim SH, Kook JH, Seo D-W, Kang MJ. The Effect of Compression Pressure on the First Layer Surface Roughness and Delamination of Metformin and Evogliptin Bilayer and Trilayer Tablets. Pharmaceuticals. 2023; 16(11):1523. https://doi.org/10.3390/ph16111523
Chicago/Turabian StyleKim, Sun Ho, Jung Han Kook, Dong-Wan Seo, and Myung Joo Kang. 2023. "The Effect of Compression Pressure on the First Layer Surface Roughness and Delamination of Metformin and Evogliptin Bilayer and Trilayer Tablets" Pharmaceuticals 16, no. 11: 1523. https://doi.org/10.3390/ph16111523