Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-Based Nanotechnologies
Abstract
:1. Introduction
2. Phenolic Compounds
3. Fields of Application of Phenolic Compounds in Dermatology
3.1. Interaction with Bacterial Cell Walls, Cell Membranes, and Synergism with Antibiotics
3.2. Interaction with Microbial DNA/RNA Polymerases and Topoisomerases, Proteases, Transcriptases, Surface Proteins (Adhesins), and Other Virulence Factors
3.3. Effects on Skin Renewal, Proliferation, Collagen, and Elastin Synthesis
3.4. Effects on Melanin Synthesis
3.5. Photosensitization
3.6. Antitumor Activity of Phenolics
3.7. Phenolics as Pro-Oxidants
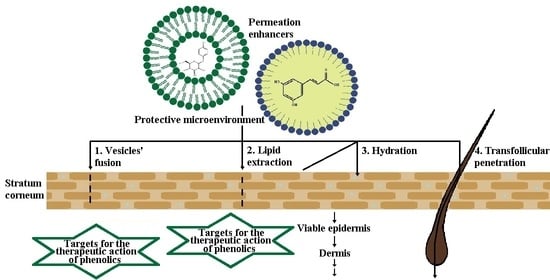
4. Dermal Drug Delivery of Phenolic Compounds
4.1. Biopharmaceutical Considerations of the Dermal Drug Delivery
4.2. Physico-Chemical Properties of Some Common Phenolic Compounds and Their Glycosides
4.3. Stability of Phenolics
5. Lipid-Based Nanotechnologies
5.1. Liposomes
5.2. Solid Lipid Nanoparticles
5.3. Nanostructured Lipid Carriers
5.4. Nanoemulsions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, J.M.; Estévez, M.; Barba, F.J.; Thirumdas, R.; Franco, D.; Munekata, P.E.S. Polyphenols: Bioaccessibility and bioavailability of bioactive components. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds, 1st ed.; Barba, F., Saraiva, J.M.A., Cravotto, G., Lorenzo, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 309–322. [Google Scholar]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mehta, J.P.; Parmar, P.H.; Vadia, S.H.; Patel, M.K.; Tripathi, C.B. In-vitro antioxidant and in-vivo anti-inflammatory activities of aerial parts of Cassia species. Arab. J. Chem. 2017, 10, S1654–S1662. [Google Scholar] [CrossRef] [Green Version]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [Green Version]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Vastag, G. Flavonoids and Phenolic Acids as Potential Natural Antioxidants. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; pp. 1–20. [Google Scholar]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Napolitano, A. Natural and Bioinspired Phenolic Compounds as Tyrosinase Inhibitors for the Treatment of Skin Hyperpigmentation: Recent Advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Przybylska-Balcerek, A.; Stuper-Szablewska, K. Phenolic acids used in the cosmetics industry as natural antioxidants. Eur. J. Med. Technol. 2019, 4, 24–32. [Google Scholar]
- Panzella, L. Natural Phenolic Compounds for Health, Food and Cosmetic Applications. Antioxidants 2020, 9, 427. [Google Scholar] [CrossRef]
- Boo, Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Schempp, C.M.; Müller, K.A.; Winghofer, B.; Schöpf, E.; Simon, J.C. Johanniskraut (Hypericum perforatum L.) Eine Pflanze mit Relevanz für die Dermatologie. Hautarzt 2002, 53, 316–321. [Google Scholar] [CrossRef]
- Arct, J.; Pytkowska, K. Flavonoids as components of biologically active cosmeceuticals. Clin. Dermatol. 2008, 26, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.; Falqué, E.; Domínguez, H. Relevance of Natural Phenolics from Grape and Derivative Products in the Formulation of Cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Naik, A.; Kalia, Y.N.; Guy, R.H. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharm. Sci. Technol. Today 2000, 3, 318–326. [Google Scholar] [CrossRef]
- Arct, J.; Gronwald, M.; Kasiura, K. Possibilities for the prediction of an active substance penetration through epidermis. IFSCC Mag. 2001, 4, 179–183. [Google Scholar]
- Arct, J.; Oborska, A.; Mojski, M.; Binkowska, A.; Świdzikowska, B. Common cosmetic hydrophilic ingredients as penetration modifiers of flavonoids. Int. J. Cosmet. Sci. 2002, 24, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Chuang, S.-Y.; Lin, Y.-K.; Lin, C.-F.; Wang, P.-W.; Chen, E.-L.; Fang, J.-Y. Elucidating the Skin Delivery of Aglycone and Glycoside Flavonoids: How the Structures Affect Cutaneous Absorption. Nutrients 2017, 9, 1304. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-drying Microencapsulation of Anthocyanins by Natural Biopolymers: A Review. Dry. Technol. 2014, 32, 509–518. [Google Scholar] [CrossRef]
- Kosović, E.; Topiař, M.; Cuřínová, P.; Sajfrtová, M. Stability testing of resveratrol and viniferin obtained from Vitis vinifera L. by various extraction methods considering the industrial viewpoint. Sci. Rep. 2020, 10, 5564. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Li, Y.; Kong, F.; Liu, K.; Si, C.; Ni, Y. Lignin-Based Nanoparticles Stabilized Pickering Emulsion for Stability Improvement and Thermal-Controlled Release of trans-Resveratrol. ACS Sustain. Chem. Eng. 2019, 7, 13497–13504. [Google Scholar] [CrossRef]
- Kumar, R.; Kaur, K.; Uppal, S.; Mehta, S.K. Ultrasound processed nanoemulsion: A comparative approach between resveratrol and resveratrol cyclodextrin inclusion complex to study its binding interactions, antioxidant activity and UV light stability. Ultrason. Sonochem. 2017, 37, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.M.; Pizzol, C.D.; Monteiro, F.B.F.; Creczynski-Pasa, T.B.; Andrade, G.P.; Ribeiro, A.O.; Perrusi, J.R. Hypericin encapsulated in solid lipid nanoparticles: Phototoxicity and photodynamic efficiency. J. Photochem. Photobiol. B Biol. 2013, 125, 146–154. [Google Scholar] [CrossRef]
- Youssef, T.; Fadel, M.; Fahmy, R.; Kassab, K. Evaluation of hypericin-loaded solid lipid nanoparticles: Physicochemical properties, photostability and phototoxicity. Pharm. Dev. Technol. 2012, 17, 177–186. [Google Scholar] [CrossRef]
- Füller, J.; Kellner, T.; Gaid, M.; Beerhues, L.; Müller-Goymann, C.C. Stabilization of hyperforin dicyclohexylammonium salt with dissolved albumin and albumin nanoparticles for studying hyperforin effects on 2D cultivation of keratinocytes in vitro. Eur. J. Pharm. Biopharm. 2018, 126, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Orth, H.C.J.; Rentel, C.; Schmidt, P.C. Isolation, Purity Analysis and Stability of Hyperforin as a Standard Material from Hypericum perforatum L. J. Pharm. Pharmacol. 1999, 51, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Koyu, H.; Haznedaroglu, M.Z. Investigation of impact of storage conditions on Hypericum perforatum L. dried total extract. J. Food Drug Anal. 2015, 23, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Park, S.N.; Lee, M.H.; Kim, S.J.; Yu, E.R. Preparation of quercetin and rutin-loaded ceramide liposomes and drug-releasing effect in liposome-in-hydrogel complex system. Biochem. Biophys. Res. Commun. 2013, 435, 361–366. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Hwang, J.; Lee, J.; Chae, S.-K.; Lee, J.-H.; Kim, J.-H.; Hwang, K.-S.; Kim, E.-C.; Park, Y.-D. Analysis and investigation of chemical stability on phenolic compounds in Zanthoxylum schinifolium-containing dentifrices. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 1685–1701. [Google Scholar] [CrossRef]
- Ramešová, Š.; Sokolová, R.; Degano, I.; Bulíčková, J.; Žabka, J.; Gál, M. On the stability of the bioactive flavonoids quercetin and luteolin under oxygen-free conditions. Anal. Bioanal. Chem. 2012, 402, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Guan, R.; Chen, X.; Liu, M.; Hao, Y.; Jiang, H. Optimized Preparation of Catechin Nanoliposomes by Orthogonal Design and Stability Study. Adv. J. Food Sci. Technol. 2014, 6, 921–925. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Natural Polymeric Compound Based on High Thermal Stability Catechin from Green Tea. Biomolecules 2020, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Kinetic Study of Catechin Stability: Effects of pH, Concentration, and Temperature. J. Agric. Food Chem. 2012, 60, 12531–12539. [Google Scholar] [CrossRef]
- Jensen, J.S.; Wertz, C.F.; O’Neill, V.A. Preformulation Stability of trans-Resveratrol and trans-Resveratrol Glucoside (Piceid). J. Agric. Food Chem. 2010, 58, 1685–1690. [Google Scholar] [CrossRef]
- Lin, C.-F.; Leu, Y.-L.; Al-Suwayeh, S.A.; Ku, M.-C.; Hwang, T.-L.; Fang, J.-Y. Anti-inflammatory activity and percutaneous absorption of quercetin and its polymethoxylated compound and glycosides: The relationships to chemical structures. Eur. J. Pharm. Sci. 2012, 47, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Křen, V. Glycoside vs. Aglycon: The Role of Glycosidic Residue in Biological Activity. In Glycoscience, 2nd ed.; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 2589–2644. [Google Scholar]
- Choi, S.-J.; Tai, B.H.; Cuong, N.M.; Kim, Y.-H.; Jang, H.-D. Antioxidative and anti-inflammatory effect of quercetin and its glycosides isolated from mampat (Cratoxylum formosum). Food Sci. Biotechnol. 2012, 21, 587–595. [Google Scholar] [CrossRef]
- Rha, C.-S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.-O. Antioxidative, Anti-Inflammatory, and Anticancer Effects of Purified Flavonol Glycosides and Aglycones in Green Tea. Antioxidants 2019, 8, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; A Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Belščak-Cvitanović, A.; Stojanović, R.; Manojlović, V.; Komes, D.; Cindrić, I.J.; Nedović, V.; Bugarski, B. Encapsulation of polyphenolic antioxidants from medicinal plant extracts in alginate–chitosan system enhanced with ascorbic acid by electrostatic extrusion. Food Res. Int. 2011, 44, 1094–1101. [Google Scholar] [CrossRef]
- Tylkowski, B.; Tsibranska, I. Polyphenols encapsulation—Application of innovation technologies to improve stability of natural products. In Microencapsulation; Giamberini, M., Prieto, S.F., Tylkowski, B., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2015; pp. 97–114. [Google Scholar]
- Kumar, R. Lipid-Based Nanoparticles for Drug-Delivery Systems. In Nanocarriers for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–284. [Google Scholar]
- Sengar, V.; Jyoti, K.; Jain, U.K.; Katare, O.P.; Chandra, R.; Madan, J. Lipid nanoparticles for topical and transdermal delivery of pharmaceuticals and cosmeceuticals. In Lipid Nanocarriers for Drug Targeting, 1st ed.; Grumezescu, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 413–436. [Google Scholar]
- Kakadia, P.; Conway, B. Lipid nanoparticles for dermal drug delivery. Curr. Pharm. Des. 2015, 21, 2823–2829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant. Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef]
- Patil, V.M.; Masand, N. Anticancer Potential of Flavonoids: Chemistry, Biological Activities, and Future Perspectives. In Studies in Natural Products Chemistry, 1st ed.; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 401–430. [Google Scholar]
- Zuiter, A.S. Proanthocyanidin: Chemistry and Biology: From Phenolic Compounds to Proanthocyanidins. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–29. [Google Scholar]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; pp. 1–28. [Google Scholar]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019, 1–17. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Gijsman, P. Polymer Stabilization. In Handbook of Environmental Degradation of Materials, 2nd ed.; Kutz, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 673–714. [Google Scholar]
- Okayama, Y. Oxidative Stress in Allergic and Inflammatory Skin Diseases. Curr. Drug Targets Inflamm. Allergy 2005, 4, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havermann, S.; Büchter, C.; Koch, K.; Wätjen, W. Role of Oxidative Stress in the Process of Carcinogenesis. In Studies on Experimental Toxicology and Pharmacology. Oxidative Stress in Applied Basic Research and Clinical Practice, 1st ed.; Roberts, S.M., Kehrer, J.P., Klotz, L.-O., Eds.; Humana Press: Totowa, NJ, USA, 2015; pp. 173–198. [Google Scholar]
- Harrison, D.; Griendling, K.K.; Landmesser, U.; Hornig, B.; Drexler, H. Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 2003, 91, 7A–11A. [Google Scholar] [CrossRef]
- Giesey, R.L.; Mehrmal, S.; Uppal, P.; Delost, G. The Global Burden of Skin and Subcutaneous Disease: A Longitudinal Analysis from the Global Burden of Disease Study From 1990–2017. SKIN J. Cutan. Med. 2021, 5, 125–136. [Google Scholar] [CrossRef]
- Karimkhani, C.; Dellavalle, R.P.; Coffeng, L.E.; Flohr, C.; Hay, R.J.; Langan, S.M.; Nsoesie, E.O.; Ferrari, A.J.; Erskine, H.E.; Silverberg, J.I.; et al. Global Skin Disease Morbidity and Mortality: An Update From the Global Burden of Disease Study 2013. JAMA Dermatol. 2017, 153, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Flohr, C.; Hay, R. Putting the burden of skin diseases on the global map. Br. J. Dermatol. 2021, 184, 189–190. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Tabassum, N.; Hamdani, M. Plants used to treat skin diseases. Pharmacogn. Rev. 2014, 8, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, A.B. Therapeutic options in the treatment of psoriasis and atopic dermatitis. J. Am. Acad. Dermatol. 2005, 53, S3–S16. [Google Scholar] [CrossRef]
- Hajar, T.; Gontijo, J.R.V.; Hanifin, J.M. New and developing therapies for atopic dermatitis. An. Bras. Dermatol. 2018, 93, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Richmond, J.M.; Harris, J.E. Immunology and Skin in Health and Disease. Cold Spring Harb. Perspect. Med. 2014, 4, a015339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dainichi, T.; Hanakawa, S.; Kabashima, K. Classification of inflammatory skin diseases: A proposal based on the disorders of the three-layered defense systems, barrier, innate immunity and acquired immunity. J. Dermatol. Sci. 2014, 76, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunter, N.V.; Teh, S.S.; Lim, Y.M.; Mah, S.H. Natural Xanthones and Skin Inflammatory Diseases: Multitargeting Mechanisms of Action and Potential Application. Front. Pharmacol. 2020, 11, 594202. [Google Scholar] [CrossRef] [PubMed]
- Schwingen, J.; Kaplan, M.; Kurschus, F.C. Review-Current Concepts in Inflammatory Skin Diseases Evolved by Transcriptome Analysis: In-Depth Analysis of Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2020, 21, 699. [Google Scholar] [CrossRef] [Green Version]
- Giang, J.; Seelen, M.A.J.; van Doorn, M.B.A.; Rissmann, R.; Prens, E.P.; Damman, J. Complement Activation in Inflammatory Skin Diseases. Front. Immunol. 2018, 9, 639. [Google Scholar] [CrossRef]
- Cetin, E.D.; Savk, E.; Uslu, M.; Eskin, M.; Karul, A. Investigation of the Inflammatory Mechanisms in Alopecia Areata. Am. J. Dermatopathol. 2009, 31, 53–60. [Google Scholar] [CrossRef]
- Woo, Y.; Lim, J.; Cho, D.; Park, H. Rosacea: Molecular Mechanisms and Management of a Chronic Cutaneous Inflammatory Condition. Int. J. Mol. Sci. 2016, 17, 1562. [Google Scholar] [CrossRef] [Green Version]
- Richmond, J.M.; Frisoli, M.L.; Harris, J.E. Innate immune mechanisms in vitiligo: Danger from within. Curr. Opin. Immunol. 2013, 25, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Neagu, M.; Constantin, C.; Caruntu, C.; Dumitru, C.; Surcel, M.; Zurac, S. Inflammation: A key process in skin tumorigenesis. Oncol. Lett. 2019, 17, 4068–4084. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, R.G., Jr.; Ferraz, C.A.A.; e Silva, M.G.; de Lavor, É.M.; Rolim, L.A.; de Lima, J.T.; Fleury, A.; Picot, L.; de Souza Siqueira Quintans, J.; Quintans, L.J., Jr.; et al. Flavonoids: Promising Natural Products for Treatment of Skin Cancer (Melanoma). In Natural Products and Cancer Drug Discovery; Badria, F.A., Ed.; Humana Press: Totowa, NJ, USA, 2017; pp. 161–210. [Google Scholar]
- Katiyar, S.K.; Afaq, F.; Perez, A.; Mukhtar, H. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 2001, 22, 287–294. [Google Scholar] [CrossRef]
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.-H.; Khan, N.; Zaid, M.A.; Mukhtar, H. Delphinidin, an Anthocyanidin in Pigmented Fruits and Vegetables, Protects Human HaCaT Keratinocytes and Mouse Skin Against UVB-Mediated Oxidative Stress and Apoptosis. J. Investig. Dermatol. 2007, 127, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Headley, C.; Stapleton, P.D.; Taylor, P.W. Synthesis and antibacterial activity of hydrolytically stable (−)-epicatechin gallate analogues for the modulation of β-lactam resistance in Staphylococcus aureus. Bioorganic Med. Chem. Lett. 2005, 15, 2633–2635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.-H.; Hu, Z.-Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of Synergy between Epigallocatechin Gallate and β-Lactams against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Sudano Roccaro, A.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-Gallate Enhances the Activity of Tetracycline in Staphylococci by Inhibiting Its Efflux from Bacterial Cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.-H.; Hu, Z.-Q.; Hara, Y.; Shimamura, T. Inhibition of Penicillinase by Epigallocatechin Gallate Resulting in Restoration of Antibacterial Activity of Penicillin Against Penicillinase-Producing Staphylococcus Aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef] [Green Version]
- Qin, R.; Xiao, K.; Li, B.; Jiang, W.; Peng, W.; Zheng, J.; Zhou, H. The Combination of Catechin and Epicatechin Gallate from Fructus Crataegi Potentiates β-Lactam Antibiotics Against Methicillin-Resistant Staphylococcus Aureus (Mrsa) In Vitro and In Vivo. Int. J. Mol. Sci. 2013, 14, 1802–1821. [Google Scholar] [CrossRef] [Green Version]
- Phan, H.T.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Vestergaard, M.C. Structure-dependent interactions of polyphenols with a biomimetic membrane system. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2670–2677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A structure–activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2751–2756. [Google Scholar] [CrossRef] [Green Version]
- Donadio, G.; Mensitieri, F.; Santoro, V.; Parisi, V.; Bellone, M.L.; De Tommasi, N.; Izzo, V.; Dal Piaz, F. Interactions with Microbial Proteins Driving the Antibacterial Activity of Flavonoids. Pharmaceutics 2021, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Ferreira, I.C.F.R.; Barros, L.; Carvalho, A.M.; Soares, G.; Henriques, M. Antibacterial Potential of Northeastern Portugal Wild Plant Extracts and Respective Phenolic Compounds. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cushnie, T.P.T.; Lamb, A.J. Assessment of the antibacterial activity of galangin against 4-quinolone resistant strains of Staphylococcus aureus. Phytomedicine 2006, 13, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Yi, Y.; Lim, M.H. Reactivity of Flavonoids Containing a Catechol or Pyrogallol Moiety with Metal-Free and Metal-Associated Amyloid-β. Bull. Korean Chem. Soc. 2020, 42, 17–24. [Google Scholar] [CrossRef]
- Barvinchenko, V.M.; Lipkovska, N.O.; Fedyanina, T.V.; Pogorelyi, V.K. Physico-chemical Properties of Supramolecular Complexes of Natural Flavonoids with Biomacromolecules. In Nanomaterials and Supramolecular Structures; Shpak, A.P., Gorbyk, P.P., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 281–291. [Google Scholar]
- Uivarosi, V.; Munteanu, A.C.; Sharma, A.; Singh Tuli, H. Metal Complexation and Patent Studies of Flavonoid. In Current Aspects of Flavonoids: Their Role in Cancer Treatment; Singh Tuli, H., Ed.; Springer: Singapore, 2019; pp. 39–89. [Google Scholar]
- Wang, S.-X.; Zhang, F.-J.; Feng, Q.-P.; Li, Y.-L. Synthesis, characterization, and antibacterial activity of transition metal complexes with 5-hydroxy-7,4-dimethoxyflavone. J. Inorg. Biochem. 1992, 46, 251–257. [Google Scholar] [CrossRef]
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 2008, 373, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanyam, K.; Varier, R.A.; Altaf, M.; Swaminathan, V.; Siddappa, N.B.; Ranga, U.; Kundu, T.K. Curcumin, a Novel p300/CREB-binding Protein-specific Inhibitor of Acetyltransferase, Represses the Acetylation of Histone/Nonhistone Proteins and Histone Acetyltransferase-dependent Chromatin Transcription. J. Biol. Chem. 2004, 279, 51163–51171. [Google Scholar] [CrossRef] [Green Version]
- Šudomová, M.; Hassan, S.T.S. Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways. Microorganisms 2021, 9, 292. [Google Scholar] [CrossRef]
- Flores, D.J.; Lee, L.H.; Adams, S.D. Inhibition of Curcumin-Treated Herpes Simplex Virus 1 and 2 in Vero Cells. Adv. Microbiol. 2016, 6, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Bernard, F.X.; Sablé, S.; Cameron, B.; Provost, J.; Desnottes, J.F.; Crouzet, J.; Blanche, F. Glycosylated flavones as selective inhibitors of topoisomerase IV. Antimicrob. Agents Chemother. 1997, 41, 992–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-H.; Otsuka, N.; Noyori, K.; Shiota, S.; Ogawa, W.; Kuroda, T.; Hatano, T.; Tsuchiya, T. Synergistic Effect of Kaempferol Glycosides Purified from Laurus nobilis and Fluoroquinolones on Methicillin-Resistant Staphylococcus aureus. Biol. Pharm. Bull. 2009, 32, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anani, K.; Adjrah, Y.; Ameyapoh, Y.; Karou, S.D.; Agbonon, A.; de Souza, C.; Gbeassor, M. Effects of hydroethanolic extracts of Balanites aegyptiaca (L.) Delile (Balanitaceae) on some resistant pathogens bacteria isolated from wounds. J. Ethnopharmacol. 2015, 164, 16–21. [Google Scholar] [CrossRef]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef]
- Wölfle, U.; Seelinger, G.; Schempp, C. Topical Application of St. John’s Wort (Hypericum perforatum). Planta Med. 2013, 80, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Feyzioğlu, B.; Demircili, M.E.; Özdemir, M.; Doğan, M.; Baykan, M.; Baysal, B. Antibacterial effect of hypericin. Afr. J. Microbiol. Res. 2013, 7, 979–982. [Google Scholar]
- Fritz, D.; Venturi, C.R.; Cargnin, S.; Schripsema, J.; Roehe, P.M.; Montanha, J.A.; von Poser, G.L. Herpes virus inhibitory substances from Hypericum connatum Lam., a plant used in southern Brazil to treat oral lesions. J. Ethnopharmacol. 2007, 113, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhang, J.; Yang, B.; Elias, P.M.; Man, M.-Q. Role of Resveratrol in Regulating Cutaneous Functions. Evid. Based Complement. Altern. Med. 2020, 2020, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.M.-Y. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem. Pharmacol. 2002, 63, 99–104. [Google Scholar] [CrossRef]
- He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef]
- Man, M.-Q.; Yang, B.; Elias, P.M. Benefits of Hesperidin for Cutaneous Functions. Evid. Based Complement. Altern. Med. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Köksal Karayıldırım, Ç. Characterization and in vitro Evolution of Antibacterial Efficacy of Novel Hesperidin Microemulsion. CBUJOS 2017, 13, 943–947. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.-W.; Im, G.J.; Chung, J.-W.; Song, J.-J. Eugenol: A Phyto-Compound Effective against Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Clinical Strain Biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, I.; Baptista-Silva, S.; Pintado, M.; Oliveira, A.L. Polyphenols: A Promising Avenue in Therapeutic Solutions for Wound Care. Appl. Sci. 2021, 11, 1230. [Google Scholar] [CrossRef]
- Thang, P.T.; Patrick, S.; Teik, L.S.; Yung, C.S. Anti-oxidant effects of the extracts from the leaves of Chromolaena odorata on human dermal fibroblasts and epidermal keratinocytes against hydrogen peroxide and hypoxanthine-xanthine oxidase induced damage. Burns 2001, 27, 319–327. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch. Dermatol. Res. 2014, 306, 601–617. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Kulma, A.; Żuk, M.; Czuj, T.; Szopa, J. The Effects of Newly Developed Linen Dressings on Decubitus Ulcers. J. Palliat. Med. 2012, 15, 146–148. [Google Scholar] [CrossRef] [Green Version]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Hou, Q.; Zhong, L.; Zhao, Y.; Li, M.; Fu, X. Bioactive Molecules for Skin Repair and Regeneration: Progress and Perspectives. Stem Cells Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eun, C.-H.; Kang, M.-S.; Kim, I.-J. Elastase/Collagenase Inhibition Compositions of Citrus unshiu and Its Association with Phenolic Content and Anti-Oxidant Activity. Appl. Sci. 2020, 10, 4838. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Cheng, H.-L.; Kuan, Y.-H.; Liang, T.-J.; Chao, Y.-Y.; Lin, H.-C. Therapeutic Potential of Luteolin on Impaired Wound Healing in Streptozotocin-Induced Rats. Biomedicines 2021, 9, 761. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Wakaizumi, M.; Ikami, T.; Saito, M. Amla (Emblica officinalis Gaertn.) extract promotes procollagen production and inhibits matrix metalloproteinase-1 in human skin fibroblasts. J. Ethnopharmacol. 2008, 119, 53–57. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Abdul Karim, A.; Azlan, A.; Ismail, A.; Hashim, P.; Abd Gani, S.S.; Zainudin, B.H.; Abdullah, N.A. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement. Altern. Med. 2014, 14, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadhel Abbas Albaayit, S.; Abba, Y.; Rasedee, A.; Abdullah, N. Effect of Clausena excavata Burm. f. (Rutaceae) leaf extract on wound healing and antioxidant activity in rats. Drug Des. Dev. Ther. 2015, 9, 3507–3518. [Google Scholar]
- Geethalakshmi, R.; Sakravarthi, C.; Kritika, T.; Arul Kirubakaran, M.; Sarada, D.V.L. Evaluation of antioxidant and wound healing potentials of Sphaeranthus amaranthoides Burm.f. Biomed. Res. Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zofia, N.-Ł.; Martyna, Z.-D.; Aleksandra, Z.; Tomasz, B. Comparison of the Antiaging and Protective Properties of Plants from the Apiaceae Family. Oxid. Med. Cell. Longev. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Dudonné, S.; Poupard, P.; Coutière, P.; Woillez, M.; Richard, T.; Mérillon, J.-M.; Vitrac, X. Phenolic Composition and Antioxidant Properties of Poplar Bud (Populus nigra) Extract: Individual Antioxidant Contribution of Phenolics and Transcriptional Effect on Skin Aging. J. Agric. Food Chem. 2011, 59, 4527–4536. [Google Scholar] [CrossRef]
- Dudonné, S.; Coutière, P.; Woillez, M.; Merillon, J.-M.; Vitrac, X. DNA macroarray study of skin aging-related genes expression modulation by antioxidant plant extracts on a replicative senescence model of human dermal fibroblasts. Phytother. Res. 2011, 25, 686–693. [Google Scholar] [CrossRef]
- Blom van Staden, A.; Lall, N. Medicinal Plants as Alternative Treatments for Progressive Macular Hypomelanosis. In Medicinal Plants for Holistic Health and Well-Being; Lall, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 145–182. [Google Scholar]
- Liu, W.-S.; Kuan, Y.-D.; Chiu, K.-H.; Wang, W.-K.; Chang, F.-H.; Liu, C.-H.; Lee, C.-H. The Extract of Rhodobacter sphaeroides Inhibits Melanogenesis through the MEK/ERK Signaling Pathway. Mar. Drugs 2013, 11, 1899–1908. [Google Scholar] [CrossRef] [Green Version]
- Parvez, S.; Kang, M.; Chung, H.-S.; Cho, C.; Hong, M.-C.; Shin, M.-K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef]
- Chai, W.-M.; Lin, M.-Z.; Wang, Y.-X.; Xu, K.-L.; Huang, W.-Y.; Pan, D.-D.; Zou, Z.-R.; Peng, Y.-Y. Inhibition of tyrosinase by cherimoya pericarp proanthocyanidins: Structural characterization, inhibitory activity and mechanism. Food Res. Int. 2017, 100, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhu, X.-F.; Ding, X.-D.; Yang, H.-B.; Qin, S.-T.; Chen, H.; Wei, S.-D. Structural features, antioxidant and tyrosinase inhibitory activities of proanthocyanidins in leaves of two tea cultivars. Int. J. Food Prop. 2016, 20, 1348–1358. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-R.; Habasi, M.; Xie, L.-Z.; Aisa, H.A. Effect of Chlorogenic Acid on Melanogenesis of B16 Melanoma Cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hariharan, V.; Toole, T.; Klarquist, J.; Mosenson, J.; Longley, B.J.; Le Poole, I.C. Topical application of bleaching phenols; in-vivo studies and mechanism of action relevant to melanoma treatment. Melanoma Res. 2011, 21, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, A.M.A.; Jeon, M.N.; Jeong, M.H.; Yang, S.Y.; Kim, Y.H. Chemical Components from the Stems of Pueraria lobata and Their Tyrosinase Inhibitory Activity. Nat. Prod. Sci. 2016, 22, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.W.; Jeong, H.O.; Lee, E.K.; Kim, S.J.; Chun, P.; Chung, H.Y.; Moon, H.R. Evaluation of Antimelanogenic Activity and Mechanism of Galangin In Silico and In Vivo. Biol. Pharm. Bull. 2018, 41, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Solimine, J.; Garo, E.; Wedler, J.; Rusanov, K.; Fertig, O.; Hamburger, M.; Atanassov, I.; Butterweck, V. Tyrosinase inhibitory constituents from a polyphenol enriched fraction of rose oil distillation wastewater. Fitoterapia 2016, 108, 13–19. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, J.H. Comparative evaluation of phenolic phytochemicals from perilla seeds of diverse species and screening for their tyrosinase inhibitory and antioxidant properties. S. Afr. J. Bot. 2019, 123, 341–350. [Google Scholar] [CrossRef]
- Tanaka, Y.; Suzuki, M.; Kodachi, Y.; Nihei, K. Molecular design of potent, hydrophilic tyrosinase inhibitors based on the natural dihydrooxyresveratrol skeleton. Carbohydr. Res. 2019, 472, 42–49. [Google Scholar] [CrossRef]
- Zuo, A.-R.; Dong, H.-H.; Yu, Y.-Y.; Shu, Q.-L.; Zheng, L.-X.; Yu, X.-Y.; Cao, S.-W. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018, 13, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespo, M.I.; Chabán, M.F.; Lanza, P.A.; Joray, M.B.; Palacios, S.M.; Vera, D.M.A.; Carpinella, M.C. Inhibitory effects of compounds isolated from Lepechinia meyenii on tyrosinase. Food Chem. Toxicol. 2019, 125, 383–391. [Google Scholar] [CrossRef]
- Akaberi, M.; Emami, S.A.; Vatani, M.; Tayarani-Najaran, Z. Evaluation of Antioxidant and Anti-Melanogenic Activity of Different Extracts of Aerial Parts of N. Sintenisii in Murine Melanoma B16F10 Cells. Iran. J. Pharm. Res. 2018, 17, 225–235. [Google Scholar]
- Demirkiran, O.; Sabudak, T.; Ozturk, M.; Topcu, G. Antioxidant and Tyrosinase Inhibitory Activities of Flavonoids from Trifolium nigrescens Subsp. petrisavi. J. Agric. Food Chem. 2013, 61, 12598–12603. [Google Scholar] [CrossRef]
- Uesugi, D.; Hamada, H.; Shimoda, K.; Kubota, N.; Ozaki, S.; Nagatani, N. Synthesis, oxygen radical absorbance capacity, and tyrosinase inhibitory activity of glycosides of resveratrol, pterostilbene, and pinostilbene. Biosci. Biotechnol. Biochem. 2017, 81, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.-M.; Zhou, Q.; Lei, T.-C.; Ding, S.-F.; Xu, S.-Z. Effects of hydroquinone and its glucoside derivatives on melanogenesis and antioxidation: Biosafety as skin whitening agents. J. Dermatol. Sci. 2009, 55, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Willemsen, K.J.; Ouwerkerk, W.; Bakker, W.J.; Ratsma, D.; Pronk, S.D.; Smit, N.P.M.; Luiten, R.M. Mechanism of action of 4-substituted phenols to induce vitiligo and antimelanoma immunity. Pigment. Cell Melanoma Res. 2019, 32, 540–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draelos, Z.D.; Deliencourt-Godefroy, G.; Lopes, L. An effective hydroquinone alternative for topical skin lightening. J. Cosmet. Dermatol. 2020, 19, 3258–3261. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, V.; Verma, P.; Naik, G. Exogenous ochronosis After Prolonged Use of Topical Hydroquinone (2%) in a 50-Year-Old Indian Female. Indian J. Dermatol. 2012, 57, 394–395. [Google Scholar] [CrossRef]
- Park, J.; Park, J.H.; Suh, H.-J.; Lee, I.C.; Koh, J.; Boo, Y.C. Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch. Dermatol. Res. 2014, 306, 475–487. [Google Scholar] [CrossRef]
- Gianfaldoni, S.; Tchernev, G.; Lotti, J.; Wollina, U.; Satolli, F.; Rovesti, M.; França, K.; Lotti, T. Unconventional Treatments for Vitiligo: Are They (Un) Satisfactory? Open Access Maced. J. Med. Sci. 2018, 6, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Rashighi, M.; Harris, J.E. Vitiligo Pathogenesis and Emerging Treatments. Dermatol. Clin. 2017, 35, 257–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivasaraun, U.V.; Sureshkumar, R.; Karthika, C.; Puttappa, N. Flavonoids as adjuvant in psoralen based photochemotherapy in the management of vitiligo/leucoderma. Med. Hypotheses 2018, 121, 26–30. [Google Scholar] [CrossRef]
- Gianfaldoni, S.; Wollina, U.; Tirant, M.; Tchernev, G.; Lotti, J.; Satolli, F.; Rovesti, M.; França, K.; Lotti, T. Herbal Compounds for the Treatment of Vitiligo: A Review. Open Access Maced. J. Med. Sci. 2018, 6, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Asawanonda, P.; Klahan, S.O. Tetrahydrocurcuminoid Cream Plus Targeted Narrowband UVB Phototherapy for Vitiligo: A Preliminary Randomized Controlled Study. Photomed. Laser Surg. 2010, 28, 679–684. [Google Scholar] [CrossRef]
- Jeong, Y.-M.; Choi, Y.-G.; Kim, D.-S.; Park, S.-H.; Yoon, J.-A.; Kwon, S.-B.; Park, E.-S.; Park, K.-C. Cytoprotective effect of green tea extract and quercetin against hydrogen peroxide-induced oxidative stress. Arch. Pharm. Res. 2005, 28, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Xu, W.; Hong, W.; Zhou, M.; Lin, F.; Fu, L.; Liu, D.; Xu, A. Quercetin attenuates the effects of H2O2 on endoplasmic reticulum morphology and tyrosinase export from the endoplasmic reticulum in melanocytes. Mol. Med. Rep. 2015, 11, 4285–4290. [Google Scholar] [CrossRef] [Green Version]
- ICH Harmonised Tripartite Guideline: Photosafety Evaluation of Pharmaceuticals S10. 2013. Available online: https://database.ich.org/sites/default/files/S10_Guideline.pdf (accessed on 14 June 2021).
- Learn, D.B.; Donald, F.P.; Sambuco, C.P. Photosafety: Current Methods and Future Direction. In A Comprehensive Guide to Toxicology in Preclinical Drug Development; Faqi, A.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 395–422. [Google Scholar]
- Li, X.; An, R.; Liang, K.; Wang, X.; You, L. Phototoxicity of traditional chinese medicine (TCM). Toxicol. Res. 2018, 7, 1012–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Park, H.; Lim, K.-M. Phototoxicity: Its Mechanism and Animal Alternative Test Methods. Toxicol. Res. 2015, 31, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.; Bonner, M.Y.; Arbiser, J.L. Use of Polyphenolic Compounds in Dermatologic Oncology. Am. J. Clin. Dermatol. 2016, 17, 369–385. [Google Scholar] [CrossRef] [Green Version]
- Jiang, A.-J.; Jiang, G.; Li, L.-T.; Zheng, J.-N. Curcumin induces apoptosis through mitochondrial pathway and caspases activation in human melanoma cells. Mol. Biol. Rep. 2015, 42, 267–275. [Google Scholar] [CrossRef]
- Abusnina, A.; Keravis, T.; Yougbaré, I.; Bronner, C.; Lugnier, C. Anti-proliferative effect of curcumin on melanoma cells is mediated by PDE1A inhibition that regulates the epigenetic integrator UHRF1. Mol. Nutr. Food Res. 2011, 55, 1677–1689. [Google Scholar] [CrossRef]
- Attoub, S.; Hassan, A.H.; Vanhoecke, B.; Iratni, R.; Takahashi, T.; Gaben, A.-M.; Bracke, M.; Awad, S.; John, A.; Kamalboor, H.A.; et al. Inhibition of cell survival, invasion, tumor growth and histone deacetylase activity by the dietary flavonoid luteolin in human epithelioid cancer cells. Eur. J. Pharmacol. 2011, 651, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhang, Y.; Deng, J.; Zeng, G.; Zhang, Y. Purified Vitexin Compound 1 Suppresses Tumor Growth and Induces Cell Apoptosis in a Mouse Model of Human Choriocarcinoma. Int. J. Gynecol. Cancer 2012, 22, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Lee, S.-H.; Song, M.H.; Yamaguchi, K.; Yoon, J.-H.; Choi, E.C.; Baek, S.J. Growth inhibition and apoptosis by (−)-epicatechin gallate are mediated by cyclin D1 suppression in head and neck squamous carcinoma cells. Eur. J. Cancer 2006, 42, 3260–3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, B.-C.; Hsu, W.-H.; Yang, J.-S.; Hsia, T.-C.; Lu, C.-C.; Chiang, J.-H.; Yang, J.-L.; Lin, C.-H.; Lin, J.-J.; Wu Suen, L.-J.; et al. Gallic Acid Induces Apoptosis via Caspase-3 and Mitochondrion-Dependent Pathways in Vitro and Suppresses Lung Xenograft Tumor Growth in Vivo. J. Agric. Food Chem. 2009, 57, 7596–7604. [Google Scholar] [CrossRef]
- Kim, G.C.; Choi, D.S.; Lim, J.S.; Jeong, H.C.; Kim, I.R.; Lee, M.H.; Park, B.S. Caspases-dependent Apoptosis in Human Melanoma Cell by Eugenol. Korean J. Anat. 2006, 39, 245–253. [Google Scholar]
- Yang, G.; Fu, Y.; Malakhova, M.; Kurinov, I.; Zhu, F.; Yao, K.; Li, H.; Chen, H.; Li, W.; Lim, D.Y.; et al. Caffeic Acid Directly Targets ERK1/2 to Attenuate Solar UV-Induced Skin Carcinogenesis. Cancer Prev. Res. 2014, 7, 1056–1066. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.B.; Chen, D.; Ping Dou, Q.; Hang Chan, T. Study of the green tea polyphenols catechin-3-gallate (CG) and epicatechin-3-gallate (ECG) as proteasome inhibitors. Bioorg. Med. Chem. 2004, 12, 3521–3527. [Google Scholar] [CrossRef]
- Pettinari, A.; Amici, M.; Cuccioloni, M.; Angeletti, M.; Fioretti, E.; Eleuteri, A.M. Effect of Polyphenolic Compounds on the Proteolytic Activities of Constitutive and Immuno-Proteasomes. Antioxid. Redox Signal. 2006, 8, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Daniel, K.G.; Chen, M.S.; Kuhn, D.J.; Landis-Piwowar, K.R.; Ping Dou, Q. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem. Pharmacol. 2005, 69, 1421–1432. [Google Scholar] [CrossRef]
- Dikshit, P.; Goswami, A.; Mishra, A.; Chatterjee, M.; Ranjan Jana, N. Curcumin induces stress response, neurite outgrowth and prevent NF-κB activation by inhibiting the proteasome function. Neurotox. Res. 2006, 9, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Mena, S.; Rodriguez, M.L.; Ponsoda, X.; Estrela, J.M.; Jäättela, M.; Ortega, A.L. Pterostilbene-Induced Tumor Cytotoxicity: A Lysosomal Membrane Permeabilization-Dependent Mechanism. PLoS ONE 2012, 7, e44524. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Chen, Y.; Tai, M.-C.; Liang, C.-M.; Lu, D.-W.; Chen, J.-T. Resveratrol inhibits transforming growth factor-β2-induced epithelial-to-mesenchymal transition in human retinal pigment epithelial cells by suppressing the Smad pathway. Drug Des. Dev. Ther. 2017, 11, 163–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.; Shen, J.; Mei, X.; Dong, H.; Li, J.; Yu, H. Hesperidin inhibits the epithelial to mesenchymal transition induced by transforming growth factor-β1 in A549 cells through Smad signaling in the cytoplasm. Braz. J. Pharm. Sci. 2019, 55, e18172. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gryko, K.; Wróblewska, A.M.; Jabłońska-Trypuć, A.; Karpowicz, D. Phenolic content, chemical composition and anti-/pro-oxidant activity of Gold Milenium and Papierowka apple peel extracts. Sci. Rep. 2020, 10, 14951. [Google Scholar] [CrossRef]
- Jomová, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3’,4’-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper(II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef] [Green Version]
- Kyselova, Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdiscip. Toxicol. 2011, 4, 173–183. [Google Scholar] [CrossRef]
- Lein, A.; Oussoren, C. Dermal. In Practical Pharmaceutics; Bouwman-Boer, Y., Fenton-May, V., Le Brun, P., Eds.; Springer: Cham, Switzerland, 2015; pp. 229–263. [Google Scholar]
- Block, L.H. Medicated topicals. In The science and practice of Pharmacy, 21st ed.; Troy, D., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 871–888. [Google Scholar]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, Ł.; Duchnik, W.; Klimowicz, A. Enhancement of the antioxidant and skin permeation properties of eugenol by the esterification of eugenol to new derivatives. AMB Express 2020, 10, 187. [Google Scholar] [CrossRef]
- Günther, A.; Makuch, E.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Pełech, R.; Klimowicz, A. Enhancement of the Antioxidant and Skin Permeation Properties of Betulin and Its Derivatives. Molecules 2021, 26, 3435. [Google Scholar] [CrossRef]
- Walters, K.A.; Lane, M.E. Dermal and Transdermal Drug Delivery Systems. In Dermal Drug Delivery, 1st ed; Ghosh, T.K., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–60. [Google Scholar]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Zhang, C.L.; Fan, J. Application of Hypericin in Tumor Treatment and Diagnosis. J. Int. Pharm. Res. Int. 2012, 39, 402–408. [Google Scholar]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozak, W.; Rachon, J.; Daśko, M.; Demkowicz, S. Selected Methods for the Chemical Phosphorylation and Thiophosphorylation of Phenols. Asian J. Org. Chem. 2018, 7, 314–323. [Google Scholar] [CrossRef]
- N’Da, D. Prodrug Strategies for Enhancing the Percutaneous Absorption of Drugs. Molecules 2014, 19, 20780–20807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, G.; Plumb, G.W.; Garcia-Conesa, M.T. Glycosylation, Esterification, and Polymerization of Flavonoids and Hydroxycinnamates: Effects on Antioxidant Properties. In Plant Polyphenols 2, 1st ed.; Gross, G.G., Hemingway, R.W., Yoshida, T., Branham, S.J., Eds.; Springer: Boston, MA, USA, 1999; pp. 483–494. [Google Scholar]
- Nowak, A.; Cybulska, K.; Makuch, E.; Kucharski, Ł.; Różewicka-Czabańska, M.; Prowans, P.; Czapla, N.; Bargiel, P.; Petriczko, J.; Klimowicz, A. In Vitro Human Skin Penetration, Antioxidant and Antimicrobial Activity of Ethanol-Water Extract of Fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 329. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Rubio, L.; Touriño, S.; Martí, M.; Barba, C.; Fernández-Campos, F.; Coderch, L.; Luís Parra, J. Antioxidative effects and percutaneous absorption of five polyphenols. Free Radic. Biol. Med. 2014, 75, 149–155. [Google Scholar] [CrossRef]
- Ng, T.B.; Wong, J.H.; Tam, C.; Liu, F.; Cheung, C.F.; Ng, C.C.W.; Tse, R.; Tse, T.F.; Chan, H. Methyl Gallate as an Antioxidant and Anti-HIV Agent. In HIV/AIDS: Oxidative Stress and Dietary Antioxidants, 1st ed.; Preedy, V.R., Watson, R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 161–168. [Google Scholar]
- Mota, F.L.; Queimada, A.J.; Pinho, S.P.; Macedo, E.A. Aqueous Solubility of Some Natural Phenolic Compounds. Ind. Eng. Chem. Res. 2008, 47, 5182–5189. [Google Scholar] [CrossRef] [Green Version]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- ChemSpider. Search and Share Chemistry. Ellagic Acid. Available online: http://www.chemspider.com/Chemical-Structure.4445149.html (accessed on 10 July 2021).
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives. A Review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kumar, M.N.V.R. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. Anal. 2006, 40, 206–210. [Google Scholar] [CrossRef]
- Simić, A.Z.; Verbić, T.Ž.; Sentić, M.N.; Vojić, M.P.; Juranić, I.O.; Manojlović, D.D. Study of ellagic acid electro-oxidation mechanism. Monatsh. Chem. 2012, 144, 121–128. [Google Scholar] [CrossRef]
- National Library of Medicine. National Center for Biotechnology Information. PubChem. Compound Summary. 4-Hydroxycinnamic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4-Hydroxycinnamic-acid#section=LogP (accessed on 11 July 2021).
- Yu, Z.; Wang, Y.; Zhu, M.; Zhou, L. Measurement and Correlation of Solubility and Thermodynamic Properties of Vinpocetine in Nine Pure Solvents and (Ethanol + Water) Binary Solvent. J. Chem. Eng. Data 2018, 64, 150–160. [Google Scholar] [CrossRef]
- Beltrán, J.L.; Sanli, N.; Fonrodona, G.; Barrón, D.; Özkan, G.; Barbosa, J. Spectrophotometric, potentiometric and chromatographic pKa values of polyphenolic acids in water and acetonitrile–water media. Anal. Chim. Acta 2003, 484, 253–264. [Google Scholar] [CrossRef]
- Paracatu, L.C.; Faria, C.M.Q.G.; Quinello, C.; Rennó, C.; Palmeira, P.; Zeraik, M.L.; de Fonseca, L.M.; Ximenes, V.F. Caffeic Acid Phenethyl Ester: Consequences of Its Hydrophobicity in the Oxidative Functions and Cytokine Release by Leukocytes. Evid. Based Complement. Altern. Med. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [Green Version]
- National Library of Medicine. National Center for Biotechnology Information. PubChem. Ferulic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ferulic-acid#section=LogP0.0 (accessed on 14 July 2021).
- FOODB. Showing Compound Chlorogenic acid (FDB002582). Available online: https://foodb.ca/compounds/FDB002582 (accessed on 14 July 2021).
- Šeruga, M.; Tomac, I. Electrochemical Behaviour of Some Chlorogenic Acids and Their Characterization in Coffee by Square-Wave Voltammetry. Int. J. Electrochem. Sci. 2014, 9, 6134–6154. [Google Scholar]
- Hansch, C.; Leo, A.; Hoekman, D.H. Exploring QSAR—Hydrophobic, Electronic, and Steric Constants, 1st ed.; American Chemical Society: Washington, DC, USA, 1995; Volume 2, p. 20. [Google Scholar]
- Cavender, F.L.; O’Donohue, J. Phenol and Phenolics. In Patty’s Toxicology, 6th ed.; Bingham, E., Cohrssen, B., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 243–349. [Google Scholar]
- Zahid, M.; Grampp, G.; Mansha, A.; Bhatti, I.A.; Asim, S. Absorption and Fluorescence Emission Attributes of a Fluorescent dye: 2,3,5,6-Tetracyano-p-Hydroquinone. J. Fluoresc. 2013, 23, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.C.; Nawas, M.I.; Poole, C.F. Evaluation of a reversed-phase column (Supelcosil LC-ABZ) under isocratic and gradient elution conditions for estimating octanol–water partition coefficients. Analyst 2003, 128, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of Aqueous Solubility Data, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 687. [Google Scholar]
- Kortum, G.; Vogel, W.; Andrussow, K. Disssociation constants of organic acids in aqueous solution. In Pure and Applied Chemistry; Burrows, H.D., Stohner, J., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 1960; Volume 1, pp. 187–536. [Google Scholar]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R.A. Experimental Determination of Octanol−Water Partition Coefficients of Quercetin and Related Flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. BioMed Res. Int. 2019, 2019, 1–18. [Google Scholar] [CrossRef]
- National Library of Medicine. National Center for Biotechnology Information. PubChem. Apigenin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Apigenin (accessed on 15 July 2021).
- de Matos, A.M.; Martins, A.; Man, T.; Evans, D.; Walter, M.; Oliveira, M.C.; López, Ó.; Fernandez-Bolaños, J.G.; Dätwyler, P.; Ernst, B.; et al. Design and Synthesis of CNS-targeted Flavones and Analogues with Neuroprotective Potential Against H2O2- and Aβ1-42-Induced Toxicity in SH-SY5Y Human Neuroblastoma Cells. Pharmaceuticals 2019, 12, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, E.C.; Menezes, P.M.N.; de Almeida, R.L.; Silva, F.S.; de Araújo Ribeiro, L.A.; de Silva, J.A.; de Oliveira, A.P.; da Cruz Araújo, E.C.; Rolim, L.A.; Nunes, X.P. Inclusion of vitexin in β-cyclodextrin: Preparation, characterization and expectorant/antitussive activities. Heliyon 2020, 6, e05461. [Google Scholar] [CrossRef]
- Chemical Book. Vitexin. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB3119208.htm (accessed on 17 July 2021).
- FOODB. Showing Compound Luteolin (FDB013255). Available online: https://foodb.ca/compounds/FDB013255 (accessed on 17 July 2021).
- Deng, S.-P.; Yang, Y.-L.; Cheng, X.-X.; Li, W.-R.; Cai, J.-Y. Synthesis, Spectroscopic Study and Radical Scavenging Activity of Kaempferol Derivatives: Enhanced Water Solubility and Antioxidant Activity. Int. J. Mol. Sci. 2019, 20, 975. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Martínez, J.M.; Sanmartin, M.; Rosés, M.; Bosch, E.; Ràfols, C. Determination of dissociation constants of flavonoids by capillary electrophoresis. Electrophoresis 2005, 26, 1886–1895. [Google Scholar] [CrossRef]
- Lončarić, A.; Lamas Castro, J.P.; Guerra, E.; Lores, M. Increasing water solubility of Quercetin by increasing the temperature. In Proceedings of the 15th Instrumental Analysis Conference/Expoquimia, Barcelona, Spain, 3–5 October 2017. [Google Scholar]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J. Food Eng. 2010, 100, 208–218. [Google Scholar] [CrossRef]
- Pedriali, C.A.; Fernandes, A.U.; de Cássia Bernusso, L.; Polakiewicz, B. The synthesis of a water-soluble derivative of rutin as an antiradical agent. Quím. Nova 2008, 31, 2147–2151. [Google Scholar] [CrossRef] [Green Version]
- Topolewski, P.; Zommer-Urbańska, S. Spectrophotometric investigation of protolytic equilibria of rutin. Microchim. Acta 1989, 97, 75–80. [Google Scholar] [CrossRef]
- Srirangam, R.; Majumdar, S. Passive asymmetric transport of hesperetin across isolated rabbit cornea. Int. J. Pharm. 2010, 394, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumdar, S.; Srirangam, R. Solubility, Stability, Physicochemical Characteristics and In Vitro Ocular Tissue Permeability of Hesperidin: A Natural Bioflavonoid. Pharm. Res. 2008, 26, 1217–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, H.; Mendes, T.; Bronze, M.R.; Simplício, A.L. Prediction of intestinal absorption and metabolism of pharmacologically active flavones and flavanones. Bioorg. Med. Chem. 2008, 16, 4009–4018. [Google Scholar] [CrossRef] [PubMed]
- Poaty, B.; Dumarçay, S.; Perrin, D. New lipophilic catechin derivatives by oxa-Pictet-Spengler reaction. Eur. Food Res. Technol. 2009, 230, 111–117. [Google Scholar] [CrossRef]
- Matsubara, T.; Wataoka, I.; Urakawa, H.; Yasunaga, H. High-Efficient Chemical Preparation of Catechinone Hair Dyestuff by Oxidation of (+)-Catechin in Water/Ethanol Mixed Solution. Sen’i Gakkaishi 2014, 70, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, L.; Li, C.; Chen, R.; Liu, C.; Chen, M. Lipophilized Epigallocatechin Gallate Derivative Exerts Anti-Proliferation Efficacy through Induction of Cell Cycle Arrest and Apoptosis on DU145 Human Prostate Cancer Cells. Nutrients 2020, 12, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, J.; Hu, J.-M.; Huang, Y.-W.; Wu, X.-Y.; Zi, C.-T.; Wang, X.-J.; Sheng, J. Synthesis and Biological Testing of Novel Glucosylated Epigallocatechin Gallate (EGCG) Derivatives. Molecules 2016, 21, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzolf-Panek, M.; Gliszczyńska-Świgło, A.; Szymusiak, H.; Tyrakowska, B. The influence of stereochemistry on the antioxidant properties of catechin epimers. Eur. Food Res. Technol. 2012, 235, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Shin, G.H.; Li, J.; Cho, J.H.; Kim, J.T.; Park, H.J. Enhancement of Curcumin Solubility by Phase Change from Crystalline to Amorphous in Cur-TPGS Nanosuspension. J. Food Sci. 2016, 81, N494–N501. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Tseng, C.-H.; Wang, P.-W.; Lu, P.-L.; Weng, Y.-H.; Yen, F.-L.; Fang, J.-Y. Pterostilbene, a Methoxylated Resveratrol Derivative, Efficiently Eradicates Planktonic, Biofilm, and Intracellular MRSA by Topical Application. Front. Microbiol. 2017, 8, 1103. [Google Scholar] [CrossRef] [Green Version]
- Robinson, K.; Mock, C.; Liang, D. Pre-formulation studies of resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; García-Carmona, F. Aggregation State and pKaValues of (E)-Resveratrol As Determined by Fluorescence Spectroscopy and UV−Visible Absorption. J. Agric. Food Chem. 2008, 56, 7600–7605. [Google Scholar] [CrossRef] [PubMed]
- Jürgenliemk, G.; Nahrstedt, A. Dissolution, solubility and cooperativity of phenolic compounds from Hypericum perforatum L. in aqueous systems. Pharmazie 2008, 58, 200–203. [Google Scholar]
- Zhang, J.; Gao, L.; Hu, J.; Wang, C.; Hagedoorn, P.-L.; Li, N.; Zhou, X. Hypericin: Source, Determination, Separation, and Properties. Sep. Purif. Rev. 2020, 1–10. [Google Scholar] [CrossRef]
- Leonhartsberger, J.G.; Falk, H. The Protonation and Deprotonation Equilibria of Hypericin Revisited. Monatsh. Chem. 2002, 133, 167–172. [Google Scholar] [CrossRef]
- National Library of Medicine. National Center for Biotechnology Information. PubChem. Hyperforin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hyperforin (accessed on 19 July 2021).
- Hadzhiiliev, V.; Dimov, D. Separate isolation of hyperforin from hypericum perforatum (St. John’s Wort) pursuant to the coefficents LOG Kow, PKa and densities of the included compounds. Trakia J. Sci. 2015, 13, 19–23. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Esparza, I.; Cimminelli, M.J.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Stability of Phenolic Compounds in Grape Stem Extracts. Antioxidants 2020, 9, 720. [Google Scholar] [CrossRef]
- Nuutila, A.M.; Kammiovirta, K.; Oksman-Caldentey, K.-M. Comparison of methods for the hydrolysis of flavonoids and phenolic acids from onion and spinach for HPLC analysis. Food Chem. 2002, 76, 519–525. [Google Scholar] [CrossRef]
- Ali, A.; Chong, C.H.; Mah, S.H.; Abdullah, L.C.; Choong, T.S.Y.; Chua, B.L. Impact of Storage Conditions on the Stability of Predominant Phenolic Constituents and Antioxidant Activity of Dried Piper betle Extracts. Molecules 2018, 23, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pignatello, R.; Pecora, T.M.G.; Cutuli, G.G.; Catalfo, A.; De Guidi, G.; Ruozi, B.; Tosi, G.; Cianciolo, S.; Musumeci, T. Antioxidant activity and photostability assessment of trans-resveratrol acrylate microspheres. Pharm. Dev. Technol. 2018, 24, 222–234. [Google Scholar] [CrossRef]
- Dodangeh, M.; Tang, R.-C.; Gharanjig, K. Improving the photostability of curcumin using functional star-shaped polyamidoamine dendrimer: Application on PET. Mater. Today Commun. 2019, 21, 100620. [Google Scholar] [CrossRef]
- Mihara, S.; Shibamoto, T. Photochemical reactions of eugenol and related compounds: Synthesis of new flavor chemicals. J. Agric. Food Chem. 1982, 30, 1215–1218. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Miolo, G.; Innocenti, G.; Caffieri, S. The Photodegradation of Quercetin: Relation to Oxidation. Molecules 2012, 17, 8898–8907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaaban, H.; Ioannou, I.; Paris, C.; Charbonnel, C.; Ghoul, M. The photostability of flavanones, flavonols and flavones and evolution of their antioxidant activity. J. Photochem. Photobiol. A Chem. 2017, 336, 131–139. [Google Scholar] [CrossRef]
- Iglesias, J.; Pazos, M.; Lois, S.; Medina, I. Contribution of Galloylation and Polymerization to the Antioxidant Activity of Polyphenols in Fish Lipid Systems. J. Agric. Food Chem. 2010, 58, 7423–7431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinardell, M.P.; Mitjans, M. Nanocarriers for Delivery of Antioxidants on the Skin. Cosmetics 2015, 2, 342–354. [Google Scholar] [CrossRef] [Green Version]
- Shade, C.W. Liposomes as Advanced Delivery Systems for Nutraceuticals. Integr. Med. 2016, 15, 33–36. [Google Scholar]
- Pierre, M.B.R.; dos Santos Miranda Costa, I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch. Dermatol. Res. 2011, 303, 607–621. [Google Scholar] [CrossRef]
- Ibaraki, H.; Kanazawa, T.; Oogi, C.; Takashima, Y.; Seta, Y. Effects of surface charge and flexibility of liposomes on dermal drug delivery. J. Drug Deliv. Sci. Technol. 2019, 50, 155–162. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malekar, S.A.; Sarode, A.L.; Bach, A.C., II; Worthen, D.R. The Localization of Phenolic Compounds in Liposomal Bilayers and Their Effects on Surface Characteristics and Colloidal Stability. AAPS PharmSciTech 2016, 17, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Jacquot, A.; Francius, G.; Razafitianamaharavo, A.; Dehghani, F.; Tamayol, A.; Linder, M.; Arab-Tehrany, E. Morphological and Physical Analysis of Natural Phospholipids-Based Biomembranes. PLoS ONE 2014, 9, e107435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoabi, A.; Touitou, E.; Margulis, K. Recent Advances in Nanomaterials for Dermal and Transdermal Applications. Colloids Interfaces 2021, 5, 18. [Google Scholar] [CrossRef]
- Figueroa-Robles, A.; Antunes-Ricardo, M.; Guajardo-Flores, D. Encapsulation of phenolic compounds with liposomal improvement in the cosmetic industry. Int. J. Pharm. 2021, 593, 120125. [Google Scholar] [CrossRef]
- Chinnagounder Periyasamy, P.; Leijten, J.C.H.; Dijkstra, P.J.; Karperien, M.; Post, J.N. Nanomaterials for the Local and Targeted Delivery of Osteoarthritis Drugs. J. Nanomater. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Particle size of liposomes influences dermal delivery of substances into skin. Int. J. Pharm. 2003, 258, 141–151. [Google Scholar] [CrossRef]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef]
- Zeb, A.; Arif, S.T.; Malik, M.; Shah, F.A.; Din, F.U.; Qureshi, O.S.; Lee, E.-S.; Lee, G.-Y.; Kim, J.-K. Potential of nanoparticulate carriers for improved drug delivery via skin. J. Pharm. Investig. 2018, 49, 485–517. [Google Scholar] [CrossRef] [Green Version]
- Park, S.N.; Jo, N.R.; Jeon, S.H. Chitosan-coated liposomes for enhanced skin permeation of resveratrol. J. Ind. Eng. Chem. 2014, 20, 1481–1485. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi-Samani, S.; Ghasemiyeh, P. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacol. Rep. 2019, 71, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Attama, A.A.; Momoh, M.A.; Builders, P.F. Lipid Nanoparticulate Drug Delivery Systems: A Revolution in Dosage Form Design and Development. In Recent Advances in Novel Drug Carrier Systems; Sezer, A.D., Ed.; IntechOpen: London, UK, 2012; pp. 107–140. [Google Scholar]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Liu, M.; Wen, J.; Sharma, M. Solid Lipid Nanoparticles for Topical Drug Delivery: Mechanisms, Dosage Form Perspectives, and Translational Status. Curr. Pharm. Des. 2020, 26, 3203–3217. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, K.; Chintamani, P. Lipid nano particulate drug delivery: An overview of the emerging trend. Pharma Innov. J. 2018, 7, 779–789. [Google Scholar]
- Wissing, S.; Lippacher, A.; Müller, R. Investigations on the occlusive properties of solid lipid nanoparticles (SLN). J. Cosmet. Sci. 2001, 52, 313–324. [Google Scholar] [PubMed]
- Kakkar, V.; Kaur, I.P.; Kaur, A.P.; Saini, K.; Singh, K.K. Topical delivery of tetrahydrocurcumin lipid nanoparticles effectively inhibits skin inflammation: In vitro and in vivo study. Drug Dev. Ind. Pharm. 2018, 44, 1701–1712. [Google Scholar] [CrossRef]
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef]
- Costa, C.P.; Barreiro, S.; Moreira, J.N.; Silva, R.; Almeida, H.; Sousa Lobo, J.M.; Silva, A.C. In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals 2021, 14, 711. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Bhise, K.; Kashaw, S.K.; Sau, S.; Iyer, A.K. Nanostructured lipid carriers employing polyphenols as promising anticancer agents: Quality by design (QbD) approach. Int. J. Pharm. 2017, 526, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Tichota, D.; Silva, A.C.; Sousa Lobo, J.M.; Amaral, M.H. Design, characterization, and clinical evaluation of argan oil nanostructured lipid carriers to improve skin hydration. Int. J. Nanomedicine 2014, 9, 3855–3864. [Google Scholar] [PubMed] [Green Version]
- Battaglia, L.; Ugazio, E. Lipid Nano- and Microparticles: An Overview of Patent-Related Research. J. Nanomater. 2019, 2019, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef]
- Puglia, C.; Lauro, M.; Offerta, A.; Crascì, L.; Micicchè, L.; Panico, A.; Bonina, F.; Puglisi, G. Nanostructured Lipid Carriers (NLC) as Vehicles for Topical Administration of Sesamol: In Vitro Percutaneous Absorption Study and Evaluation of Antioxidant Activity. Planta Med. 2016, 83, 398–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, C.H.; Basri, M.; Ismail, R.; Lau, H.L.N.; Tejo, B.A.; Kanthimathi, M.S.; Hassan, H.A.; Choo, Y. Effect of compositions in nanostructured lipid carriers (NLC) on skin hydration and occlusion. Int. J. Nanomedicine 2013, 8, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech. 2014, 5, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che Marzuki, N.H.; Wahab, R.A.; Abdul Hamid, M. An overview of nanoemulsion: Concepts of development and cosmeceutical applications. Biotechnol. Biotechnol. Equip. 2019, 33, 779–797. [Google Scholar] [CrossRef] [Green Version]
- Nastiti, C.; Ponto, T.; Abd, E.; Grice, J.; Benson, H.; Roberts, M. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Ugur Kaplan, A.B.; Cetin, M.; Orgul, D.; Taghizadehghalehjoughi, A.; Hacımuftuoglu, A.; Hekimoglu, S. Formulation and in vitro evaluation of topical nanoemulsion and nanoemulsion-based gels containing daidzein. J. Drug Deliv. Sci. Technol. 2019, 52, 189–203. [Google Scholar] [CrossRef]
- Su, R.; Fan, W.; Yu, Q.; Dong, X.; Qi, J.; Zhu, Q.; Zhao, W.; Wu, W.; Chen, Z.; Li, Y.; et al. Size-dependent penetration of nanoemulsions into epidermis and hair follicles: Implications for transdermal delivery and immunization. Oncotarget 2017, 8, 38214–38226. [Google Scholar] [CrossRef] [Green Version]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation, development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Hu, J.; Sui, H.; Zhao, Q.; Zhang, X.; Wang, W. Enhanced skin permeation of glabridin using eutectic mixture-based nanoemulsion. Drug Deliv. Transl. Res. 2017, 7, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef] [Green Version]
- Ghanbarzadeh, S.; Hariri, R.; Kouhsoltani, M.; Shokri, J.; Javadzadeh, Y.; Hamishehkar, H. Enhanced stability and dermal delivery of hydroquinone using solid lipid nanoparticles. Colloids Surf. B. Biointerfaces 2015, 136, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.-H.; Choi, M.-K.; Kim, D.-D. Formulation of Liposome for topical delivery of arbutin. Arch. Pharm. Res. 2006, 29, 1187–1192. [Google Scholar] [CrossRef]
- de Lourdes Reis Giada, M. Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-Gonzalez, J.A., Ed.; IntechOpen: London, UK, 2013; pp. 87–112. [Google Scholar]
- Mostafa, M.; Alaaeldin, E.; Aly, U.F.; Sarhan, H.A. Optimization and Characterization of Thymoquinone-Loaded Liposomes with Enhanced Topical Anti-inflammatory Activity. AAPS PharmSciTech 2018, 19, 3490–3500. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. Int. Sch. Res. Notices 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Daré, R.G.; Costa, A.; Nakamura, C.V.; Truiti, M.C.T.; Ximenes, V.F.; Lautenschlager, S.O.S.; Sarmento, B. Evaluation of lipid nanoparticles for topical delivery of protocatechuic acid and ethyl protocatechuate as a new photoprotection strategy. Int. J. Pharm. 2020, 582, 119336. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Mukherjee, P.K.; Bahadur, S.; Biswas, R. Enhanced permeability of ferulic acid loaded nanoemulsion based gel through skin against UVA mediated oxidative stress. Life Sci. 2015, 141, 202–211. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Perera, A.D.L.C.; Karunaratne, V.; Amaratunga, G.A.J.; Karunaratne, D.N. Improved Delivery of Caffeic Acid through Liposomal Encapsulation. J. Nanomater. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Garg, A.; Singh, S. Targeting of eugenol-loaded solid lipid nanoparticles to the epidermal layer of human skin. Nanomedicine 2014, 9, 1223–1238. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Huang, Y.-B.; Fang, J.-W.; Fu, Y.-S.; Wu, P.-C. Preparation and Characterization of Naringenin-Loaded Elastic Liposomes for Topical Application. PLoS ONE 2015, 10, e0131026. [Google Scholar] [CrossRef] [Green Version]
- Durán, N.; Costa, A.F.; Stanisic, D.; Bernardes, J.S.; Tasic, L. Nanotoxicity and Dermal Application of Nanostructured Lipid Carrier Loaded with Hesperidin from Orange Residue. J. Phys. Conf. Ser. 2019, 1323, 012021. [Google Scholar] [CrossRef]
- Nemitz, M.C.; von Poser, G.L.; Teixeira, H.F. In vitro skin permeation/retention of daidzein, genistein and glycitein from a soybean isoflavone rich fraction-loaded nanoemulsions and derived hydrogels. J. Drug Deliv. Sci. Technol. 2019, 51, 63–69. [Google Scholar] [CrossRef]
- Chou, T.-H.; Liang, C.-H. The Molecular Effects of Aloe-Emodin (AE)/Liposome-AE on Human Nonmelanoma Skin Cancer Cells and Skin Permeation. Chem. Res. Toxicol. 2009, 22, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Campani, V.; Marchese, D.; Pitaro, M.T.; Pitaro, M.; Grieco, P.; De Rosa, G. Development of a liposome-based formulation for vitamin K1 nebulization on the skin. Int. J. Nanomedicine 2014, 9, 1823–1832. [Google Scholar] [PubMed] [Green Version]
- Pleguezuelos-Villa, M.; Nácher, A.; Hernández, M.J.; Ofelia Vila Buso, M.A.; Ruiz Sauri, A.; Díez-Sales, O. Mangiferin nanoemulsions in treatment of inflammatory disorders and skin regeneration. Int. J. Pharm. 2019, 564, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Gugleva, V.; Zasheva, S.; Hristova, M.; Andonova, V. Topical use of resveratrol: Technological aspects. Pharmacia 2020, 67, 89–94. [Google Scholar] [CrossRef]
- Gokce, E.; Korkmaz, E.; Dellera, E.; Sandri, G.; Bonferoni, M.C.; Ozer, O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int. J. Nanomedicine 2012, 7, 1841–1850. [Google Scholar] [CrossRef] [Green Version]
- Sirerol, J.A.; Feddi, F.; Mena, S.; Rodriguez, M.L.; Sirera, P.; Aupí, M.; Pérez, S.; Asensi, M.; Ortega, A.; Estrela, J.M. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Radic. Biol. Med. 2015, 85, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Singh Hallan, S.; Sguizzato, M.; Pavoni, G.; Baldisserotto, A.; Drechsler, M.; Mariani, P.; Esposito, E.; Cortesi, R. Ellagic Acid Containing Nanostructured Lipid Carriers for Topical Application: A Preliminary Study. Molecules 2020, 25, 1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, M.S.; Magalhães, M.C.; Oliveira, R.; Sousa-Lobo, J.M.; Almeida, I.F. Trends in the Use of Botanicals in Anti-Aging Cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef] [PubMed]
- SESDERMA Listening to Your Skin. Available online: https://www.sesderma.com/eu_en/home (accessed on 23 July 2021).
- M.Y.R. COSMETICS SOLUTION Innovative Organization. Available online: https://www.myrcosmeticssolution.com/ (accessed on 23 July 2021).
- VITACOS Corporation. Available online: http://www.vitacos.co.kr/english/main.php?m1=44&m2=46&m3=&board_mode=list&board_no=19&board_search_keyword=&board_page=1&board_search_head_word=&board_mode=view&board_no=20 (accessed on 23 July 2021).

| Phenolic Class | Phenolic Subclass | Phenolic Compound | Molecular Weight (g/mol) | Partition Coefficient (Log P) | Solubility in Water at 25 °C (mg/mL) | Ionizable Groups with Corresponding pKa Values |
|---|---|---|---|---|---|---|
| Simple phenols and derivatives | Phenolic acids (hydroxybenzoic acid derivatives) |  Gallic acid | 170.12 | −0.28 [208] | 14.7 [209] | pKa1 = 4.51 pKa2 = 8.7 pKa3 = 11.4 pKa4 > 13 [210] |
 Ellagic acid (ellagitannin, dimer of gallic acid) | 390.12 | 1.37 [211] | 0.0097 [212,213] (at 37 °C) | pKa1 = 5.42 pKa2 = 6.76 [214] | ||
| Phenolic acids (hydroxycinnamic acid derivatives) |  p-Coumaric acid | 164.05 | 1.46 [215] | 0.01 [216] | pKa1 = 4.92 pKa2 = 9.28 [217] | |
 Caffeic acid | 180.16 | 1.15 [218] | 0.98 [209] | pKa1 = 4.83 pKa2 = 8.90 pKa3 = 10.28 [217] | ||
 Ferulic acid | 194.18 | 1.51 [219] | 0.78 [209] | pKa1 = 4.66 pKa2 = 9.09 [217] | ||
 Chlorogenic acid | 354.31 | −0.75 [218] | 3.44 * [220] | pKa1 = 3.50 pKa2 = 8.42 pKa3 = 11.00 [221] | ||
| Other simple phenols |  Hydroquinone | 110.11 | 0.59 [222] | 7.20 [223] | pKa1 = 9.85 pKa2 = 11.40 [224] | |
 Eugenol | 164.20 | 2.49 [225] | 2.46 [226] | pKa = 10.19 [227] | ||
| Flavonoids | Flavones |  Apigenin | 270.05 | 2.92 [228] | 0.00135 [229] | pKa1 = 7.12 pKa2 = 8.10 [230] |
 Vitexin (Apigenin-8-C-glucoside) | 432.38 | 0.1 * [231] | 0.0762 [232] | pKa1 = 6.27 * [233] ** | ||
 Luteolin | 286.24 | 3.22 [228] | 0.14 * [234] | pKa1 = 6.57 * [234] ** | ||
| Flavonols |  Kaemferol | 286.23 | 3.11 [228] | 0.113 [235] (at 30 °C) | pKa1 = 6.96 pKa2 = 8.78 pKa3 = 10.60 [236] | |
 Quercetin | 302.24 | 1.82 [228] | 0.0004 [237] −0.002 [238] | pKa1 = 7.10 pKa2 = 9.09 pKa3 = 11.12 [236] | ||
 Rutin (Quercetin-3-O-rutinoside) | 610.52 | 0.76 [22] | 0.125 [239] | pKa1 = 2.92 pKa2 = 6.72 pKa3 = 8.26 pKa4 = 12.57 [240] | ||
| Flavanones |  Hesperitin | 302.27 | 2.9 [241] | 0.01572 [241] | pKa1 = 7.55 * pKa2 = 8.50 * pKa3 = 9.65 * | |
 Hesperidin (Hesperitin- 7-(6-O-(alpha-l-rhamnopyranosyl)-beta-d-glucopyranosyl) | 610.19 | 1.78 [242] | 0.00495 [242] | pKa1 = 10.0 pKa2 > 11.5 [243] | ||
| Flavan-3-ols |  Catechin | 290.26 | 0.41 [244] | 7.66 [245] | pKa1 = 8.68 pKa2 = 9.70 pKa3 = 11.55 [236] | |
 Epigallocatechin gallate | 458.37 | 0.46 [246] | 16.05 [247] | pKa1 = 7.75 pKa2 = 8.00 [248] | ||
| Curcuminoids |  Curcumin (keto form) | 368.38 | 3.0 [249] | 0.0006 [250] | pKa1 = 7.7–8.5 pKa2 = 8.5–10.4 pKa3 = 9.5–10.7 [249] | |
| Stilbenes |  Resveratrol | 228.25 | 3.09 * [251] | 0.05 [252] | pKa1 = 8.8 pKa2 = 9.8 pKa3 = 11.4 [253] | |
| Anthraquinones |  Hypericin | 504.44 | 3.43 [254] | Practically insoluble in water [255] ** | pKa1 = 2.00 pKa2 = 11.00 [256] | |
| Phloroglucinols |  Hyperforin | 536.78 | 13.17 [257] | 2.34 × 10−12 [257] | pKa = 6.32 * [258] | |
| Main Class | Active Agent | Technological/Biopharmaceutical Issue | Lipid-Based Nanosystem/ Lipid Carrier | Obtained Results | References |
|---|---|---|---|---|---|
| Simple phenols and derivatives | Hydroquinone | Tendency to oxidation; hydrophilic structure hindering its topical application; side-effects due to systemic absorption | SLNs/Precirol® ATO5 |
| [311] |
| Arbutin | Highly hydrophilic compound; limited skin permeation | Liposomes/ Soybean phosphatidylcholine; cholesterol |
| [312,313] | |
| Thymoquinone (benzo-quinone) | Thermo/photosensitivity; hydrophobicity | Liposomes/ Phospholipon 90H®; cholesterol |
| [314] | |
| Protocatechuic acid; Ethyl protocatechuate (phenolic acid and derivative) | Sparingly hydrosolubilty (1:50); skin irritating properties; photosensitivity | SLNs/ Precirol ATO®5; NLCs/ Miglyol®810 N: Precirol ATO®5 3:7 |
| [315,316] | |
| Ferulic acid (phenolic acid) | Poor water solubility; low stability | Nanoemulsion/ Isostearyl isosearate |
| [317] | |
| Caffeic acid (hydroxyl-cinnamic acid) | Limited skin permeation | Liposomes/ egg phosphatidyl-choline; cholesterol |
| [318] | |
| Eugenol (Clove oil); (phenylpropene) | Predisposition to oxidation | SLNs/Stearic acid, Compritol® |
| [319] | |
| Flavanoids | Naringin (flavanone) | Limited aqueous solubility; poor oral bioavailability | Liposomes/ Epikuron-200; cholesterol, Tween 80 |
| [320] |
| Hesperidin (flavanone glycoside) | Poor aqueous solubility and bioavailability | NLCs/ Cupuaçu butter, buriti oil |
| [321] | |
| Isoflavone-aglycon-rich fraction (genistein, daidzein, glycitein) | Limited aqueous solubility | Nanoemulsion/ Egg lecithin (Lipoid E-80®), medium-chain triglycerides |
| [322] | |
| Anthraquinones | Aloe -emodin | Hydrophobic compound, crystallizes in water | Liposomes/ Hydrogenated soybean phosphatidyl-choline, cholesterol |
| [323] |
| Naphthoquinones | Vitamin K1 (phylloquinone) | Highly lipophilic compound; photosensitivity | Liposomes/ Soy phosphatidyl-choline, α-tocopherol |
| [324] |
| Xanthones | Mangiferin | Poor water solubility (0.111 mg/mL); low bioavailability | Nanoemulsion/ Almond oil, Lipoid ®S75 |
| [325] |
| Stilbenes | Resveratrol | Low aqueous solubility; photosensitivity | SLNs/ Compritol 888 ATO NLCs/ Compritol 888 ATO; Miglyol oil |
| [326,327] |
| Pterostilbene | None; favorable characteristics compared to resveratrol (i.e., increased lipophilicity, membrane permeability and bioavailability) | Liposomes/ Lecithin |
| [328] | |
| Tannins | Ellagic acid | Low aqueous solubility and permeability | NLCs/ Tristearin, Miglyol oil |
| [329] |
| Brand | Product | Phenolic Compounds | Lipid-Based Nanosystem | Benefits |
|---|---|---|---|---|
| Sesderma [331] | Sodyses Repair gel | Resveratrol, quercetin | Liposomes |
|
| Factor G Renew Rejuvenating serum | Quercetin, pterostilbene | Liposomes |
| |
| Hidroquin Whitening gel | Ferulic acid, Arbutin | Liposomes |
| |
| Reti Age Eye contour gel | Pterostilbene | Liposomes |
| |
| Kojicol Plus (+Kojic acid) Skin lightener cream | α-Arbutin, | Liposomes |
| |
| M.Y.R. [332] | Curcumin liposome Melasma and acne cream | Curcumin | Liposomes |
|
| Vitacos [333] | NanoVital Vitanics Whitening essence | Arbutin | Nanoemulsion |
|
| Dr. Theiss Medipharma Cosme-tics [298] | Olivenöl Anti Falten Pflege-konzentrat | Olea europaea oil | NLCs |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugleva, V.; Ivanova, N.; Sotirova, Y.; Andonova, V. Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-Based Nanotechnologies. Pharmaceuticals 2021, 14, 837. https://doi.org/10.3390/ph14090837
Gugleva V, Ivanova N, Sotirova Y, Andonova V. Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-Based Nanotechnologies. Pharmaceuticals. 2021; 14(9):837. https://doi.org/10.3390/ph14090837
Chicago/Turabian StyleGugleva, Viliana, Nadezhda Ivanova, Yoana Sotirova, and Velichka Andonova. 2021. "Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-Based Nanotechnologies" Pharmaceuticals 14, no. 9: 837. https://doi.org/10.3390/ph14090837