Molecular Identification of Trypanosome Diversity in Domestic Animals Reveals the Presence of Trypanosoma brucei gambiense in Historical Foci of Human African Trypanosomiasis in Gabon
Abstract
:1. Introduction
2. Results
2.1. Occurrence of Trypanosomes
2.2. Diversity and Prevalence of Each Parasite
2.3. Prevalence of the Mixed Infections
2.4. Overview of Some African Countries Reported Isolation of T. b. gambiense in Domestic Animals
3. Discussion
3.1. Diversity of Trypanosomes
3.2. Prevalence of Trypanosoma spp. Infection
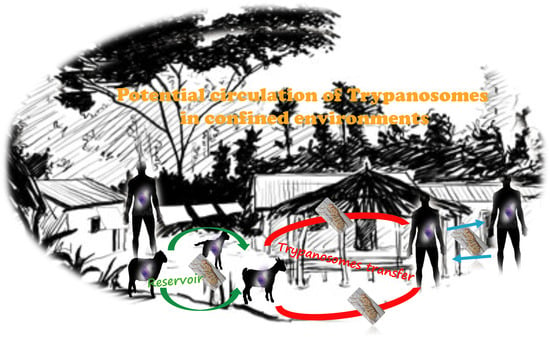
3.3. The Necessity of One Health Approach Strategies
4. Materials and Methods
4.1. Study Area and Samples Collection
4.2. DNA Extraction and Polymerase Chain Reaction (PCR)
4.3. Molecular Amplification for Identification of Different Trypanosome Species
4.4. Molecular Identification of T. b. gambiense
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, P.G. Update on human African trypanosomiasis (sleeping sickness). J. Neurol. 2019, 266, 2334–2337. [Google Scholar] [CrossRef] [PubMed]
- Krafsur, E.; Maudlin, I. Tsetse fly evolution, genetics and the trypanosomiases—A review. Infect. Genet. Evol. 2018, 64, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, S.; Buscher, P.; Lehane, M.; Solano, P.; Van Den Abbeele, J. Human African trypanosomiasis control: Achievements and challenges. PLoS Negl. Trop. Dis. 2017, 11, e0005454. [Google Scholar] [CrossRef] [PubMed]
- WHO. Lignes directrices provisoires de l’OMS pour le traitement de la trypanosomiase humaine africaine à T. b. gambiense. 201. In WHO Interim Guidelines for the Treatment of Gambiense Human African Trypanosomiasis; Licence: CCBY-NC-SA 3.0 IGO; Organisation Mondiale de la Santé: Genève, Switzerland, 2019. [Google Scholar]
- Hasker, E.; Lutumba, P.; Chappuis, F.; Kande, V.; Potet, J.; De Weggheleire, A.; Kambo, C.; Depoortere, E.; Pécoul, B.; Boelaert, M. Human African trypanosomiasis in the Democratic Republic of the Congo: A looming emergency? PLoS Negl. Trop. Dis. 2012, 6, e1950. [Google Scholar] [CrossRef]
- Franco, J.R.; Simarro, P.P.; Diarra, A.; Ruiz-Postigo, J.A.; Jannin, J.G. The journey towards elimination of gambiense human African trypanosomiasis: Not far, nor easy. Parasitology 2014, 141, 748–760. [Google Scholar] [CrossRef]
- Fevre, E.M.; Wissmann, B.V.; Welburn, S.C.; Lutumba, P. The burden of human African trypanosomiasis. PLoS Negl. Trop. Dis. 2008, 2, e333. [Google Scholar] [CrossRef]
- Liana, Y.A.; Shaban, N.; Mlay, G.; Phibert, A. African trypanosomiasis dynamics: Modelling the effects of treatment, education, and vector trapping. In. J. Math. Sci. 2020, 2020, 3690472. [Google Scholar] [CrossRef]
- Lindner, A.K.; Lejon, V.; Chappuis, F.; Seixas, J.; Kazumba, L.; Barrett, M.P.; Mwamba, E.; Erphas, O.; Akl, E.A.; Villanueva, G. New WHO guidelines for treatment of gambiense human African trypanosomiasis including fexinidazole: Substantial changes for clinical practice. Lancet Infect. Dis. 2020, 20, e38–e46. [Google Scholar] [CrossRef]
- Picozzi, K.; Fèvre, E.; Odiit, M.; Carrington, M.; Eisler, M.C.; Maudlin, I.; Welburn, S.C. Sleeping sickness in Uganda: A thin line between two fatal diseases. BMJ 2005, 331, 1238–1241. [Google Scholar] [CrossRef]
- Courtin, F.; Kaba, D.; Rayaisse, J.B.; Solano, P.; Torr, S.J.; Shaw, A.P. The cost of tsetse control using ‘Tiny Targets’ in the sleeping sickness endemic forest area of Bonon in Côte d’Ivoire: Implications for comparing costs across different settings. PLoS Negl. Trop. Dis. 2022, 16, e0010033. [Google Scholar] [CrossRef]
- Mahamat, M.H.; Peka, M.; Rayaisse, J.B.; Rock, K.S.; Toko, M.A.; Darnas, J.; Brahim, G.M.; Alkatib, A.B.; Yoni, W.; Tirados, I.; et al. Adding tsetse control to medical activities contributes to decreasing transmission of sleeping sickness in the Mandoul focus (Chad). PLoS Negl. Trop. Dis. 2017, 11, e0005792. [Google Scholar] [CrossRef] [PubMed]
- Ndung’u, J.M.; Boulangé, A.; Picado, A.; Mugenyi, A.; Mortensen, A.; Hope, A.; Mollo, B.G.; Bucheton, B.; Wamboga, C.; Waiswa, C.; et al. Trypa-NO! contributes to the elimination of gambiense human African trypanosomiasis by combining tsetse control with “screen, diagnose and treat” using innovative tools and strategies. PLoS Negl. Trop. Dis. 2020, 14, e0008738. [Google Scholar] [CrossRef] [PubMed]
- Wamboga, C.; Matovu, E.; Bessell, P.R.; Picado, A.; Biéler, S.; Ndung’u, J.M. Enhanced passive screening and diagnosis for gambiense human African trypanosomiasis in north-western Uganda—Moving towards elimination. PLoS ONE 2017, 12, e0186429. [Google Scholar] [CrossRef]
- Holt, H.; Selby, R.; Mumba, C.; Napier, G.; Guitian, J. Assessment of animal African trypanosomiasis (AAT) vulnerability in cattle-owning communities of sub-Saharan Africa. Parasit Vectors 2016, 9, 53. [Google Scholar] [CrossRef]
- Morrison, L.J.; Vezza, L.; Rowan, T.; Hope, J.C. Animal African trypanosomiasis: Time to increase focus on clinically relevant parasite and host species. Trends Parasitol 2016, 32, 599–607. [Google Scholar] [CrossRef]
- Squarre, D.; Hayashida, K.; Gaithuma, A.; Chambaro, H.; Kawai, N.; Moonga, L.; Namangala, B.; Sugimoto, C.; Yamagishi, J. Diversity of trypanosomes in wildlife of the Kafue ecosystem, Zambia. Int. J. Parasitol. Parasites Wildl. 2020, 12, 34–41. [Google Scholar] [CrossRef]
- Maganga, G.D.; Mavoungou, J.-F.; N’dilimabaka, N.; Kinga, I.C.M.; Mvé-Ondo, B.; Mombo, I.M.; Ngoubangoye, B.; Cossic, B.; Okouyi, C.S.M.; Souza, A. Molecular identification of trypanosome species in trypanotolerant cattle from the south of Gabon. Parasite 2017, 24, 53. [Google Scholar] [CrossRef]
- Maganga, G.D.; Boundenga, L.; Ologui-Minkue-Edzo, E.J.; Kombila, L.B.; Mebaley, T.G.N.; Kumulungui, B.; Mavoungou, J.F. Frequency and diversity of trypanosomes in sheep and goats from Mongo County in South Gabon, Central Africa. Vet. World 2020, 13, 2502. [Google Scholar] [CrossRef]
- Marsela, M.; Hayashida, K.; Nakao, R.; Chatanga, E.; Gaithuma, A.K.; Naoko, K.; Musaya, J.; Sugimoto, C.; Yamagishi, J. Molecular identification of trypanosomes in cattle in Malawi using PCR methods and nanopore sequencing: Epidemiological implications for the control of human and animal trypanosomiases. Parasite 2020, 27, 46. [Google Scholar] [CrossRef]
- Deborggraeve, S.; Koffi, M.; Jamonneau, V.; Bonsu, F.A.; Queyson, R.; Simarro, P.P.; Herdewijn, P.; Buscher, P. Molecular analysis of archived blood slides reveals an atypical human Trypanosoma infection. Diagn. Microbiol. Infect. Dis. 2008, 61, 428–433. [Google Scholar] [CrossRef]
- Haridy, F.M.; El-Metwally, M.T.; Khalil, H.H.; Morsy, T.A. Trypanosoma evansi in dromedary camel: With a case report of zoonosis in greater Cairo, Egypt. J. Egypt Soc. Parasitol. 2011, 41, 65–76. [Google Scholar] [PubMed]
- Truc, P.; Büscher, P.; Cuny, G.; Gonzatti, M.I.; Jannin, J.; Joshi, P.; Juyal, P.; Lun, Z.-R.; Mattioli, R.; Pays, E. Atypical human infections by animal trypanosomes. PLoS Negl. Trop. Dis. 2013, 7, e2256. [Google Scholar] [CrossRef] [PubMed]
- Njiokou, F.; Nimpaye, H.; Simo, G.; Njitchouang, G.; Asonganyi, T.; Cuny, G.; Herder, S. Domestic animals as potential reservoir hosts of Trypanosoma brucei gambiense in sleeping sickness foci in Cameroon. Parasite 2010, 17, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Hamill, L.C.; Kaare, M.T.; Welburn, S.C.; Picozzi, K. Domestic pigs as potential reservoirs of human and animal trypanosomiasis in Northern Tanzania. Parasit Vectors 2013, 6, 322. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.; Kutz, S.J.; Smith, A. Parasite zoonoses and wildlife: Emerging issues. Int. J. Environ. Res. Public Health 2009, 6, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Funk, S.; Nishiura, H.; Heesterbeek, H.; Edmunds, W.J.; Checchi, F. Identifying transmission cycles at the human-animal interface: The role of animal reservoirs in maintaining gambiense human african trypanosomiasis. PLoS Comput. Biol. 2013, 9, e1002855. [Google Scholar] [CrossRef]
- Simon, F.; Mura, M.; Pagès, F.; Morand, G.; Truc, P.; Louis, F.; Gautret, P. Urban transmission of human African trypanosomiasis, Gabon. Emerg Infect. Dis. 2012, 18, 165. [Google Scholar] [CrossRef]
- Iroungou, B.A.; Boundenga, L.; Guignali Mangouka, L.; Bivigou-Mboumba, B.; Nzenze, J.R.; Maganga, G.D. Human African trypanosomiasis in two historical foci of the estuaire province, gabon: A case report. SAGE Open Med. Case Rep. 2020, 8, 2050313X20959890. [Google Scholar] [CrossRef]
- Mbang Nguema, O.A.; Mavoungou, J.F.; Mawili-Mboumba, D.P.; Zinga Koumba, R.C.; Bouyou-Akotet, M.K.; M’Batchi, B. Inventory of potential vectors of trypanosoma and infection rate of the Tsetse fly in the National Park of Ivindo, Gabon. Afr. Health Sci. 2015, 15, 762–767. [Google Scholar] [CrossRef]
- Kohagne Tongué, L.; Gounoue Kamkuimo, R.; Mengue M’eyi, P.; Kaba, D.; Louis, F.J.; Mimpfoundi, R. Entomological survey in the historical focus of human African trypanosomiasis of Bendje (Gabon). Parasite 2011, 18, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Morens, D.M.; Fauci, A.S. Emerging Pandemic Diseases: How We Got to COVID-19. Cell 2020, 182, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.T.; de Roode, J.C.; Fenton, A. Why infectious disease research needs community ecology. Science 2015, 349, 1259504. [Google Scholar] [CrossRef] [PubMed]
- Desquesnes, M.; Michel, J.-F.; De La Rocque, S.; Solano, P.; Millogo, L.; Bengaly, Z.; Sididé, I. Enquête parasitologique et sérologique (Elisa-indirect) sur les trypanosomoses des bovins dans la zone de Sidéradougou, Burkina Faso. Rev. Elev. Méd. Vét. Pays. Trop. 1999, 52, 223–232. [Google Scholar] [CrossRef]
- Reifenberg, J.M.; Solano, P.; Duvallet, G.; Cuisance, D.; Simpore, J.; Cuny, G. Molecular characterization of trypanosome isolates from naturally infected domestic animals in Burkina, Faso. Vet. Parasitol. 1997, 71, 251–262. [Google Scholar] [CrossRef]
- Nimpaye, H.; Njiokou, F.; Njine, T.; Njitchouang, G.; Cuny, G.; Herder, S.; Asonganyi, T.; Simo, G. Trypanosoma vivax, T. congolense “forest type” and T. simiae: Prevalence in domestic animals of sleeping sickness foci of Cameroon. Parasite J. Société Française Parasitol. 2011, 18, 171. [Google Scholar] [CrossRef]
- Nkinin, S.W.; Njiokou, F.; Penchenier, L.; Grébaut, P.; Simo, G.; Herder, S. Characterization of Trypanosoma brucei s.l. subspecies by isoenzymes in domestic pigs from the Fontem sleeping sickness focus of Cameroon. Acta tropica 2002, 81, 225–232. [Google Scholar] [CrossRef]
- Simo, G.; Asonganyi, T.; Nkinin, S.W.; Njiokou, F.; Herder, S. High prevalence of Trypanosoma brucei gambiense group 1 in pigs from the Fontem sleeping sickness focus in Cameroon. Vet. Parasitol. 2006, 139, 57–66. [Google Scholar] [CrossRef]
- Vourchakbé, J.; Tiofack, Z.A.A.; Kante, T.S.; Mpoame, M.; Simo, G. Molecular identification of Trypanosoma brucei gambiense in naturally infected pigs, dogs and small ruminants confirms domestic animals as potential reservoirs for sleeping sickness in Chad. Parasite 2020, 27, 63. [Google Scholar] [CrossRef]
- Vourchakbé, J.; Tiofack, A.A.Z.; Mbida, M.; Simo, G. Trypanosome infections in naturally infected horses and donkeys of three active sleeping sickness foci in the south of Chad. Parasit. Vectors 2020, 13, 323. [Google Scholar] [CrossRef]
- Scott, C.M.; Frézil, J.-L.; Toudic, A.; Godfrey, D. The sheep as a potential reservoir of human trypanosomiasis in the Republic of the Congo. Trans. R Soc. Trop. Med. Hyg. 1983, 77, 397–401. [Google Scholar] [CrossRef]
- Truc, P.; Mathieu-Daudé, F.; Tibayrenc, M. Multilocus isozyme identification of Trypanosoma brucei stocks isolated in central Africa: Evidence for an animal reservoir of sleeping sickness in Congo. Acta Trop. 1991, 49, 127–135. [Google Scholar] [CrossRef]
- N’Djetchi, M.K.; Ilboudo, H.; Koffi, M.; Kaboré, J.; Kaboré, J.W.; Kaba, D.; Courtin, F.; Coulibaly, B.; Fauret, P.; Kouakou, L. The study of trypanosome species circulating in domestic animals in two human African trypanosomiasis foci of Cote d’Ivoire identifies pigs and cattle as potential reservoirs of Trypanosoma brucei gambiense. PLoS Negl. Trop. Dis. 2017, 11, e0005993. [Google Scholar] [CrossRef] [PubMed]
- Traoré, B.M.; Koffi, M.; N’Djetchi, M.K.; Kaba, D.; Kaboré, J.; Ilboudo, H.; Ahouty, B.A.; Koné, M.; Coulibaly, B.; Konan, T.; et al. Free-ranging pigs identified as a multi-reservoir of Trypanosoma brucei and Trypanosoma congolense in the Vavoua area, a historical sleeping sickness focus of Côte d’Ivoire. PLoS Negl. Trop. Dis. 2021, 15, e0010036. [Google Scholar] [CrossRef] [PubMed]
- Kouadio, I.K.; Sokouri, D.; Koffi, M.; Konaté, I.; Ahouty, B.; Koffi, A.; N’Guetta, S.P. Molecular characterization and prevalence of Trypanosoma species in cattle from a northern livestock area in Côte d’Ivoire. Open J. Vet. Med. 2014, 4, 314. [Google Scholar] [CrossRef]
- Cordon-Obras, C.; Berzosa, P.; Ndong-Mabale, N.; Bobuakasi, L.; Buatiche, J.N.; Ndongo-Asumu, P.; Benito, A.; Cano, J. Trypanosoma brucei gambiense in domestic livestock of Kogo and Mbini foci (Equatorial Guinea). Trop. Med. Int. Health 2009, 14, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Cordon-Obras, C.; Rodriguez, Y.F.; Fernandez-Martinez, A.; Cano, J.; Ndong-Mabale, N.; Ncogo-Ada, P.; Ndongo-Asumu, P.; Aparicio, P.; Navarro, M.; Benito, A.; et al. Molecular evidence of a Trypanosoma brucei gambiense sylvatic cycle in the human african trypanosomiasis focii of Equatorial Guinea. Front. Microbiol. 2015, 6, 765. [Google Scholar] [CrossRef]
- Cordon-Obras, C.; García-Estébanez, C.; Ndong-Mabale, N.; Abaga, S.; Ndongo-Asumu, P.; Benito, A.; Cano, J. Screening of Trypanosoma brucei gambiense in domestic livestock and tsetse flies from an insular endemic focus (Luba, Equatorial Guinea). PLoS Negl. Trop. Dis. 2010, 4, e704. [Google Scholar] [CrossRef]
- Büscher, P.; Bart, J.M.; Boelaert, M.; Bucheton, B.; Cecchi, G.; Chitnis, N.; Courtin, D.; Figueiredo, L.M.; Franco, J.R.; Grébaut, P.; et al. Do Cryptic Reservoirs Threaten Gambiense-Sleeping Sickness Elimination? Trends. Parasitol. 2018, 34, 197–207. [Google Scholar] [CrossRef]
- Rodrigues, C.M.F.; Garcia, H.A.; Sheferaw, D.; Rodrigues, A.C.; Pereira, C.L.; Camargo, E.P.; Teixeira, M.M.G. Genetic diversity of trypanosomes pathogenic to livestock in tsetse flies from the Nech Sar National Park in Ethiopia: A concern for tsetse suppressed area in Southern Rift Valley? Infect. Genet. Evol. 2019, 69, 38–47. [Google Scholar] [CrossRef]
- Kargbo, A.; Ebiloma, G.U.; Ibrahim, Y.K.E.; Chechet, G.D.; Jeng, M.; Balogun, E.O. Epizootiology and molecular identification of trypanosome species in Livestock ruminants in the Gambia. Acta Parasitol. 2022, 67, 130–142. [Google Scholar] [CrossRef]
- Nakayima, J.; Nakao, R.; Alhassan, A.; Mahama, C.; Afakye, K.; Sugimoto, C. Molecular epidemiological studies on animal trypanosomiases in Ghana. Parasit. Vectors 2012, 5, 217. [Google Scholar] [CrossRef] [PubMed]
- Apaatah, F.; Osae, M.; Nwaefuna, E.; Aboagye-Antwi, F.; Egyir-Yawson, A.; Bimi, L. Trypanosome prevalence in pigs and tsetse flies from selected areas of Jomoro district of the western region of Ghana. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100444. [Google Scholar] [CrossRef] [PubMed]
- Jamonneau, V.; Truc, P.; Grébaut, P.; Herder, S.; Ravel, S.; Solano, P.; De Meeus, T. Trypanosoma brucei gambiense Group 2: The Unusual Suspect. Trend. Parasitol. 2019, 35, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Getahun, M.N.; Villinger, J.; Bargul, J.L.; Orone, A.; Ngiela, J.; Ahuya, P.O.; Muema, J.M.; Saini, R.K.; Torto, B.; Masiga, D.K. Molecular characterization of pathogenic African trypanosomes in biting flies and camels in surra-endemic areas outside the tsetse fly belt in Kenya. BioRxiv 2020. [Google Scholar] [CrossRef]
- Gibson, W. The origins of the trypanosome genome strains Trypanosoma brucei brucei TREU 927, T. b. gambiense DAL 972, T. vivax Y486 and T. congolense IL3000. Parasit. Vectors 2012, 5, 71. [Google Scholar] [CrossRef]
- Habeeb, I.F.; Chechet, G.D.; Kwaga, J.K.P. Molecular identification and prevalence of trypanosomes in cattle distributed within the Jebba axis of the River Niger, Kwara state, Nigeria. Parasit. Vectors 2021, 14, 560. [Google Scholar] [CrossRef]
- Enwezor, F.; Kugama, M.; Emmanuel, R.; Olanrewaju, T.; Yarnap, J.; Bizi, R.; David, K.; Ezebuiro, O.; Yusuf, R.; Abubakar, S. Investigation of livestock for presence of Trypanosoma brucei gambiense in Tafa Local Government Area of Niger State, Nigeria. Sci. World J. 2019, 14, 39–44. [Google Scholar]
- Karshima, S.N.; Lawal, I.A.; Bata, S.I.; Barde, I.J.; Adamu, P.V.; Salihu, A.; Dross, P.N.; Obalisa, A. Animal reservoirs of Trypanosoma brucei gambiense around the old Gboko sleeping sickness focus in Nigeria. J. Parasitol. Res. 2016, 2016, 2656121. [Google Scholar] [CrossRef]
- Umeakuana, P.U.; Gibson, W.; Ezeokonkwo, R.C.; Anene, B.M. Identification of Trypanosoma brucei gambiense in naturally infected dogs in Nigeria. Parasit. Vectors 2019, 12, 420. [Google Scholar] [CrossRef]
- Musaya, J.; Chisi, J.; Senga, E.; Nambala, P.; Maganga, E.; Matovu, E.; Enyaru, J. Polymerase chain reaction identification of Trypanosoma brucei rhodesiense in wild tsetse flies from Nkhotakota Wildlife Reserve, Malawi. Malawi. Med. J. 2017, 29, 11–15. [Google Scholar] [CrossRef]
- Mahmood, M.S.; Alobaidii, W. Detection of Trypanosoma in sheep and goat in Mosul City. J. Agric. Vet. Sci. 2021, 14, 48–54. [Google Scholar]
- Meisner, J.; Kato, A.; Lemerani, M.; Miaka, E.M.; Taban, A.I.; Wakefield, J.; Rowhani-Rahbar, A.; Pigott, D.; Mayer, J.; Rabinowitz, P.M. The effect of livestock density on Trypanosoma brucei gambiense and T. b. rhodesiense: A causal inference-based approach. medRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Maina, N.W.; Oberle, M.; Otieno, C.; Kunz, C.; Maeser, P.; Ndung’u, J.M.; Brun, R. Isolation and propagation of Trypanosoma brucei gambiense from sleeping sickness patients in south Sudan. Trans. R Soc. Trop. Med. Hyg. 2007, 101, 540–546. [Google Scholar] [CrossRef]
- Mugittu, K.N.; Silayo, R.S.; Majiwa, P.A.; Kimbita, E.K.; Mutayoba, B.M.; Maselle, R. Application of PCR and DNA probes in the characterisation of trypanosomes in the blood of cattle in farms in Morogoro, Tanzania. Vet. Parasitol. 2001, 94, 177–189. [Google Scholar] [CrossRef]
- Simwango, M.; Ngonyoka, A.; Nnko, H.J.; Salekwa, L.P.; Ole-Neselle, M.; Kimera, S.I.; Gwakisa, P.S. Molecular prevalence of trypanosome infections in cattle and tsetse flies in the Maasai Steppe, northern Tanzania. Parasit. Vectors 2017, 10, 507. [Google Scholar] [CrossRef]
- Kimaro, E.G.; Toribio, J.; Gwakisa, P.; Mor, S.M. Occurrence of trypanosome infections in cattle in relation to season, livestock movement and management practices of Maasai pastoralists in Northern Tanzania. Vet. Parasitol. Reg. Stud. Rep. 2018, 12, 91–98. [Google Scholar] [CrossRef]
- Ruiz, J.P.; Nyingilili, H.S.; Mbata, G.H.; Malele, I.I. The role of domestic animals in the epidemiology of human African trypanosomiasis in Ngorongoro conservation area, Tanzania. Parasit. Vectors 2015, 8, 510. [Google Scholar] [CrossRef]
- Nhamitambo, N.; Kimera, S.; Gwakisa, P. Molecular identification of trypanosome species in cattle of the Mikumi human/livestock/wildlife interface areas. Tanzania. J. Infect. Dis. Epidemiol. 2017, 3, 029. [Google Scholar] [CrossRef]
- Schares, G.; Mehlitz, D. Sleeping sickness in Zaire: A nested polymerase chain reaction improves the identification of Trypanosoma (Trypanozoon) brucei gambiense by specific kinetoplast DNA probes. Trop. Med. Int. Health 1996, 1, 59–70. [Google Scholar] [CrossRef]
- Matovu, E.; Mugasa, C.M.; Waiswa, P.; Kitibwa, A.; Boobo, A.; Ndung’u, J.M. Haemoparasitic infections in cattle from a Trypanosoma brucei rhodesiense sleeping sickness endemic district of eastern Uganda. Trop. Med. Infect. Dis. 2020, 5, 24. [Google Scholar] [CrossRef]
- Balyeidhusa, A.S.; Kironde, F.A.; Enyaru, J.C. Apparent lack of a domestic animal reservoir in Gambiense sleeping sickness in northwest Uganda. Vet. Parasitol. 2012, 187, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Simo, G.; Silatsa, B.; Flobert, N.; Lutumba, P.; Mansinsa, P.; Madinga, J.; Manzambi, E.; De Deken, R.; Asonganyi, T. Identification of different trypanosome species in the mid-guts of tsetse flies of the Malanga (Kimpese) sleeping sickness focus of the Democratic Republic of Congo. Parasit. Vectors 2012, 5, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waiswa, C.; Olaho-Mukani, W.; Katunguka-Rwakishaya, E. Domestic animals as reservoirs for sleeping sickness in three endemic foci in south–eastern Uganda. Ann. Trop. Med. Parasitol. 2003, 97, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Meisner, J.; Barnabas, R.V.; Rabinowitz, P.M. A mathematical model for evaluating the role of trypanocide treatment of cattle in the epidemiology and control of Trypanosoma brucei rhodesiense and T. b. gambiense sleeping sickness in Uganda. Parasite Epidemiol. Control 2019, 5, e00106. [Google Scholar] [CrossRef]
- Hamill, L.; Picozzi, K.; Fyfe, J.; von Wissmann, B.; Wastling, S.; Wardrop, N.; Selby, R.; Acup, C.A.; Bardosh, K.L.; Muhanguzi, D.; et al. Evaluating the impact of targeting livestock for the prevention of human and animal trypanosomiasis, at village level, in districts newly affected with T. b. rhodesiense in Uganda. Infect. Dis. Poverty 2017, 6, 16. [Google Scholar] [CrossRef]
- Lisulo, M.; Sugimoto, C.; Kajino, K.; Hayashida, K.; Mudenda, M.; Moonga, L.; Ndebe, J.; Nzala, S.; Namangala, B. Determination of the prevalence of African trypanosome species in indigenous dogs of Mambwe district, eastern Zambia, by loop-mediated isothermal amplification. Parasit. Vectors 2014, 7, 19. [Google Scholar] [CrossRef]
- Haji, I.J.; Sugimoto, C.; Kajino, K.; Malele, I.; Simukoko, H.; Chitambo, H.; Namangala, B. Determination of the prevalence of trypanosome species in cattle from Monduli district, northern Tanzania, by loop mediated isothermal amplification. Trop. Anim. Health Prod. 2015, 47, 1139–1143. [Google Scholar] [CrossRef]
- Nyimba, P.; Komba, E.; Sugimoto, C.; Namangala, B. Prevalence and species distribution of caprine trypanosomosis in Sinazongwe and Kalomo districts of Zambia. Vet. Parasitol. 2015, 210, 125–130. [Google Scholar] [CrossRef]
- Truc, P.; Cuny, G. Distribution and spread of human African trypanosomiasis: Value of genetic identification of the trypanosomes. Med. Trop. 2001, 61, 433–436. [Google Scholar]
- Auty, H.; Anderson, N.E.; Picozzi, K.; Lembo, T.; Mubanga, J.; Hoare, R.; Fyumagwa, R.D.; Mable, B.; Hamill, L.; Cleaveland, S.; et al. Trypanosome diversity in wildlife species from the serengeti and Luangwa Valley ecosystems. PLoS Negl. Trop. Dis. 2012, 6, e1828. [Google Scholar] [CrossRef]
- Ng’ayo, M.O.; Njiru, Z.K.; Kenya, E.U.; Muluvi, G.M.; Osir, E.O.; Masiga, D.K. Detection of trypanosomes in small ruminants and pigs in western Kenya: Important reservoirs in the epidemiology of sleeping sickness? Kinetoplastid. Biol. Dis. 2005, 4, 19. [Google Scholar]
- Simo, G.; Njitchouang, G.R.; Njiokou, F.; Cuny, G.; Asonganyi, T. Genetic characterization of Trypanosoma brucei circulating in domestic animals of the Fontem sleeping sickness of Cameroon. Microbes Infect. 2012, 14, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Majekodunmi, A.O.; Fajinmi, A.; Dongkum, C.; Picozzi, K.; Thrusfield, M.V.; Welburn, S.C. A longitudinal survey of African animal trypanosomiasis in domestic cattle on the Jos Plateau, Nigeria: Prevalence, distribution and risk factors. Parasit Vectors 2013, 6, 239. [Google Scholar] [CrossRef] [PubMed]
- Torr, S.; Mangwiro, T. Interactions between cattle and biting flies: Effects on the feeding rate of tsetse. Med. Vet. Entomol. 2000, 14, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Torr, S.J.; Mangwiro, T.; Hall, D.R. The effects of host physiology on the attraction of tsetse (Diptera: Glossinidae) and Stomoxys (Diptera: Muscidae) to cattle. Bull. Entomol. Res. 2006, 96, 71–84. [Google Scholar] [CrossRef]
- Simukoko, H.; Marcotty, T.; Phiri, I.; Geysen, D.; Vercruysse, J.; Van den Bossche, P. The comparative role of cattle, goats and pigs in the epidemiology of livestock trypanosomiasis on the plateau of eastern Zambia. Vet. Parasitol. 2007, 147, 231–238. [Google Scholar] [CrossRef]
- Von Wissmann, B.; Machila, N.; Picozzi, K.; Fèvre, E.M.; Bronsvoort, B.M.d.C.; Handel, I.G.; Welburn, S.C. Factors associated with acquisition of human infective and animal infective trypanosome infections in domestic livestock in western Kenya. PLoS Negl. Trop. Dis. 2011, 5, e941. [Google Scholar] [CrossRef]
- Biryomumaisho, S.; Rwakishaya, E.K.; Melville, S.E.; Cailleau, A.; Lubega, G.W. Livestock trypanosomosis in Uganda: Parasite heterogeneity and anaemia status of naturally infected cattle, goats and pigs. Parasitol. Res. 2013, 112, 1443–1450. [Google Scholar] [CrossRef]
- Ngomtcho, S.C.H.; Weber, J.S.; Bum, E.N.; Gbem, T.T.; Kelm, S.; Achukwi, M.D. Molecular screening of tsetse flies and cattle reveal different Trypanosoma species including T. grayi and T. theileri in northern Cameroon. Parasit. Vectors 2017, 10, 631. [Google Scholar] [CrossRef]
- Taylor, K.; Authie, E.M.-L. 18 Pathogenesis of Animal Trypanosomiasis. Trypanosomiases 2004, 331, 331–353. [Google Scholar]
- Van den Bossche, P.; Chitanga, S.; Masumu, J.; Marcotty, T.; Delespaux, V. Virulence in Trypanosoma congolense Savannah subgroup. A comparison between strains and transmission cycles. Parasite Immunol. 2011, 33, 456–460. [Google Scholar] [CrossRef]
- Truc, P.; Nzoumbou-Boko, R.; Desquesnes, M.; Semballa, S.; Vincendeau, P. Atypical human trypanosomoses. Med. Sante Trop. 2014, 24, 249–252. [Google Scholar] [CrossRef]
- Kohagne, T.; M’eyi, M.; Mimpfoundi, R.; Louis, J. Entomological patterns in the human African trypanosomiasis focus of Komo Mondah, Gabon. Afr. Health Sci. 2010, 10, 341–348. [Google Scholar]
- Kohagne Tongué, L.; Mengue M’eyi, P.; Mimpfoundi, R.; Louis, F. Régime alimentaire des glossines et diversité des espèces de trypanosomes dans un foyer actif de trypanosomose humaine africaine au Gabon. Bull. Soc. Pathol. Exot. 2010, 103, 264–271. [Google Scholar] [CrossRef]
- Mehlitz, D.; Molyneux, D.H. The elimination of Trypanosoma brucei gambiense? Challenges of reservoir hosts and transmission cycles: Expect the unexpected. Parasite Epidemiol. Control. 2019, 6, e00113. [Google Scholar] [CrossRef]
- Franco, A.O.; Gomes, M.G.; Rowland, M.; Coleman, P.G.; Davies, C.R. Controlling malaria using livestock-based interventions: A one health approach. PLoS ONE 2014, 9, e101699. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, S.; Rowland, M. Control of zoophilic malaria vectors by applying pyrethroid insecticides to cattle. Trop. Med. Int. Health 1999, 4, 481–486. [Google Scholar] [CrossRef]
- Pareyn, M.; Kochora, A.; Van Rooy, L.; Eligo, N.; Vanden Broecke, B.; Girma, N.; Merdekios, B.; Wegayehu, T.; Maes, L.; Caljon, G.; et al. Feeding behavior and activity of Phlebotomus pedifer and potential reservoir hosts of Leishmania aethiopica in southwestern Ethiopia. PLoS Negl. Trop. Dis. 2020, 14, e0007947. [Google Scholar] [CrossRef]
- Franco, J.R.; Cecchi, G.; Paone, M.; Diarra, A.; Grout, L.; Kadima Ebeja, A.; Simarro, P.P.; Zhao, W.; Argaw, D. The elimination of human African trypanosomiasis: Achievements in relation to WHO road map targets for 2020. PLoS Negl. Trop. Dis. 2022, 16, e0010047. [Google Scholar] [CrossRef]
- Radwanska, M.; Claes, F.; Magez, S.; Magnus, E.; Perez-Morga, D.; Pays, E.; Büscher, P. Novel primer sequences for polymerase chain reaction-based detection of Trypanosoma brucei gambiense. Am. J. Trop. Med. 2002, 67, 289–295. [Google Scholar] [CrossRef]
- Morrison, L.J.; Tait, A.; McCormack, G.; Sweeney, L.; Black, A.; Truc, P.; Likeufack, A.C.; Turner, C.M.; MacLeod, A. Trypanosoma brucei gambiense Type 1 populations from human patients are clonal and display geographical genetic differentiation. Infect. Genet. Evol. 2008, 8, 847–854. [Google Scholar] [CrossRef]


| Dogs | Goats | Sheep | Prevalence (%) (Infected/Examined) | |||||
|---|---|---|---|---|---|---|---|---|
| Province | City | Examined | Infected (%) | Examined | Infected (%) | Examined | Infected (%) | |
| Estuaire | Libreville | 14 | 3 (21) | 12 | 2 (17) | 19 | 4 (21) | 20 ± 11.69 (9/45) |
| Cocobeach | 10 | 4 (40) | 23 | 6 (26) | 21 | 3 (14) | 24 ± 11.40 (13/54) | |
| Ntoum | 05 | 1 (20) | 19 | 3 (16) | 13 | 5 (38) | 24 ± 13.82 (9/37) | |
| Ogooue-Maritime | Port-Gentil | 11 | 3 (27) | - | - | 27 ± 18.53 (3/11) | ||
| Moyen-Ogooue | Lambarene | 07 | 0 (0) | 20 | 2 (10) | 19 | 2 (11) | 9 ± 8.14 (4/46) |
| Haut-Ogooue | Bongoville | 04 | 2 (50) | 10 | 0 (0) | 17 | 3 (18) | 16 ± 12.95 (5/31) |
| Franceville | 05 | 0 (0) | 34 | 6 (18) | 11 | 4 (36) | 20 ± 11.09 (10/50) | |
| Total prevalence | 56 | 13 (23.21 ± 11.06) | 118 | 19 (16.1 ± 6.63) | 100 | 21 (21 ± 7.98) | 19 ± 4.68 (53/274) | |
| Parasite Species/Subspecies | Dogs n (Pravalence) | Goats n (Prevalence) | Sheep n (Prevalence) | Global Infection Rate (%) |
|---|---|---|---|---|
| T. b. brucei | 5 (9) | 4 (3) | - | 3.28 |
| T. b. gambiense | 5 (9) | - | 2 (2) | 2.55 |
| T. congolense | 3 (5) | 2 (2) | 4 (4) | 3.28 |
| T. vivax | - | 8 (7) | - | 2.91 |
| T. simiae | - | 5 (4) | 8 (8) | 4.74 |
| T. simiae Tsavo | - | 3 (3) | - | 1.09 |
| T. theileri | - | - | 7 (7) | 2.55 |
| Total | 13/56 (23.2) | 22/118 (18.6) | 21/100 (21) |
| Host Species | Percent Mixed Infections (Co-Infected/Infected) | Parasites (Number of Observations) |
|---|---|---|
| Goats | 36.84% (7/19) | T. b. brucei-T. simiae (1) |
| T. vivax-T. simiae (1) | ||
| T. simae-T. simiae Tsavo (3) | ||
| T. vivax-T. congolense (2) | ||
| Dogs | 15.38% (2/13) | T. b. brucei-T. b. gambiense (1) |
| T. b. brucei-T. congolense (1) | ||
| Sheep | 9.52% (2/21) | T. simiae-T. b. gambiens (1) |
| T. simiae-T. theileri (1) |
| Round | Primer Sequences | Parasites Species/Subspecies | Gene Amplified | Product Size (bp) |
|---|---|---|---|---|
| PCR1 | ITS 1: 5′-GATTACGTCCCTGCCATTTG-3′ | T. congolense | 18S ARNr gene | 1408–1501 |
| ITS 2: 5′-TTGTTCGCTATCGGTCTTCC- 3′ | T. brucei brucei | 1215 | ||
| PCR2 | ITS 3: 5′-GGAAGCAAAAGTCGTAACAAGG-3′ | T. theileri | 998 | |
| ITS 4: 5′-TGTTTTCTTTTCCTCCGCTG-3′ | T. simiae Tsavo | 951 | ||
| T. simiae | 847 | |||
| T. vivax | 620 | |||
| PCR1 | TgSGP-1: 5′–GCTGCTGTGTTCGGAGAGC–3′ | T. b. gambiense | 270 | |
| TgSGP-2: 5–GCC ATCGTGCTTGCCGCTC–3 | ||||
| PCR1 | TgsGP-s: 5′–TCAGAC AGG GCT GTA ATA GCAAGC-3′ | |||
| TgsGP-as: 5–GGGCTCCTGCCTCAATTGCTGCA–3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boundenga, L.; Mombo, I.M.; Augustin, M.-O.; Barthélémy, N.; Nzassi, P.M.; Moukodoum, N.D.; Rougeron, V.; Prugnolle, F. Molecular Identification of Trypanosome Diversity in Domestic Animals Reveals the Presence of Trypanosoma brucei gambiense in Historical Foci of Human African Trypanosomiasis in Gabon. Pathogens 2022, 11, 992. https://doi.org/10.3390/pathogens11090992
Boundenga L, Mombo IM, Augustin M-O, Barthélémy N, Nzassi PM, Moukodoum ND, Rougeron V, Prugnolle F. Molecular Identification of Trypanosome Diversity in Domestic Animals Reveals the Presence of Trypanosoma brucei gambiense in Historical Foci of Human African Trypanosomiasis in Gabon. Pathogens. 2022; 11(9):992. https://doi.org/10.3390/pathogens11090992
Chicago/Turabian StyleBoundenga, Larson, Illich Manfred Mombo, Mouinga-Ondeme Augustin, Ngoubangoye Barthélémy, Patrice Makouloutou Nzassi, Nancy D. Moukodoum, Virginie Rougeron, and Franck Prugnolle. 2022. "Molecular Identification of Trypanosome Diversity in Domestic Animals Reveals the Presence of Trypanosoma brucei gambiense in Historical Foci of Human African Trypanosomiasis in Gabon" Pathogens 11, no. 9: 992. https://doi.org/10.3390/pathogens11090992