A Search for Anti-Naegleria fowleri Agents Based on Competitive Exclusion Behavior of Microorganisms in Natural Aquatic Environments
Abstract
:1. Introduction
2. Results and Discussion
2.1. Anti-N. fowleri Effect of the Cell-Free Culture Fluids of Aquatic Bacteria
2.2. Identification of the Aquatic Bacteria That Produced Anti-N. fowleri Products
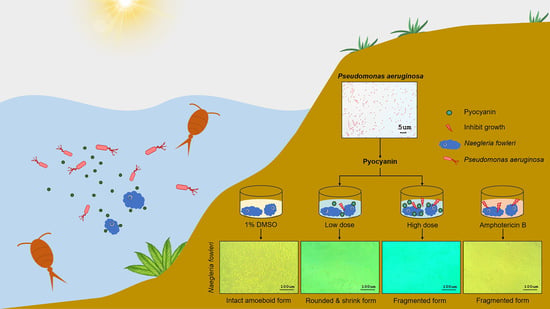
2.3. Effect of Pyocyanin on N. fowleri
3. Materials and Methods
3.1. N. fowleri, Bacteria, and Culture Conditions
3.2. Chemical Agents
3.3. Preparation of the Cell-Free Culture Supernatants of the Environmental Bacteria
3.4. N. fowleri Inhibition Assay
3.5. Enumeration of N. fowleri Viable Cells and Cell Proliferation Assay
3.6. Identification of the KP-01, KP-14, and KP-15 Bacteria
3.7. Anti-N. fowleri Activity of Pyocyanin
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.L. Pathogenesis of pathogenic Naegleria amoeba. Folia Parasitol. 1979, 26, 195–200. [Google Scholar]
- Martinez, A.J. Free-Living Amebas: Natural History, Prevention, Diagnosis, Pathology, and Treatment of Disease; CRC Press Inc.: Boca Raton, FL, USA, 1985. [Google Scholar]
- Grace, E.; Asbill, S.; Virga, K. Naegleria fowleri: Pathogenesis, diagnosis, and treatment options. Antimicrob. Agents Chemother. 2015, 59, 6677–6681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, M.; Carter, R.F. Acute pyogenic meningitis probably due to Acanthamoeba sp.: A preliminary report. Br. Med. J. 1965, 2, 740–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorsch, M.M.; Cameron, A.S.; Robinson, B.S. The epidemiology and control of primary amoebic meningoencephalitis with particular reference to South Australia. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 372–377. [Google Scholar] [CrossRef]
- Gharpure, R.; Bliton, J.; Goodman, A.; Ali, I.K.M.; Yoder, J.; Cope, J.R. Epidemiology and clinical characteristics of primary amebic meningoencephalitis caused by Naegleria fowleri: A global review. Clin. Infect. Dis 2020. [Google Scholar] [CrossRef]
- Maciver, S.K.; Piñero, J.E.; Lorenzo-Morales, J. Is Naegleria fowleri an emerging parasite? Trends Parasitol. 2020, 36, 19–28. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Review of clinical presentations in Thai patients with primary amoebic meningoencephalitis. Med. Gen. Med. 2004, 6, 2. [Google Scholar]
- Ma, P.; Visvesvara, G.S.; Martinez, A.J.; Theodore, F.H.; Daggett, M.; Sawyer, T.K. Naegleria and Acanthamoeba infection. Rev. Infect. Dis. 1990, 12, 490–513. [Google Scholar] [CrossRef]
- Chow, F.C.; Glaser, C.A. Emerging and reemerging neurologic infections. Neurohospitalist 2014, 4, e173–e184. [Google Scholar] [CrossRef] [Green Version]
- Gautam, P.L. A rare case of survival from primary amebic meningoencephalitis. Indian J. Crit. Care Med. 2012, 16, 34–36. [Google Scholar] [PubMed] [Green Version]
- Schuster, F.L.; Visvesvara, G.S. Opportunistic amoebae: Challenges in prophylaxis and treatment. Drug Resist. Updates 2004, 7, e41–e51. [Google Scholar] [CrossRef] [PubMed]
- Yoder, J.S.; Straif-Bourgeois, S.; Roy, S.L.; Moore, T.A.; Visvesvara, G.S.; Ratard, R.C.; Hill, V.R.; Wilson, J.D.; Linscott, A.J.; Crager, R.; et al. Primary amebic meningoencephalitis deaths associated with sinus irrigation using contaminated tap water. Clin. Infect. Dis. 2012, 55, e79–e85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruenchit, P.; Reamtong, R.; Siripanichgon, K.; Chaicumpa, W.; Diraphat, P. New facet of non-O1/non-O139 Vibrio cholerae hemolysin A: A competitive factor in the ecological niche. FEMS Microbiol. Ecol. 2018, 94, fix113. [Google Scholar] [CrossRef] [Green Version]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, M.A.; Kainz, K.; Carmona-Gutierrez, D.; Madeo, F. Microbial wars: Competition in ecological niches and within the microbiome. Microbial. Cell 2018, 5, 215–219. [Google Scholar] [CrossRef]
- Duerkop, B.A.; Varga, J.; Chandler, J.R.; Peterson, S.B.; Herman, J.P.; Churchill, M.E.; Parsek, M.R.; Nierman, W.C.; Greenberg, E.P. Quorum-sensing control of antibiotic synthesis in Burkholderia thailandensis. J. Bacteriol. 2009, 191, 3909–3918. [Google Scholar] [CrossRef] [Green Version]
- Ngamdee, W.; Tandhavanant, S.; Wikraiphat, C.; Reamtong, O.; Wuthiekanun, V.; Salje, J.; Low, D.A.; Peacock, S.J.; Chantratita, N. Competition between Burkholderia pseudomallei and B. thailandensis. BMC Microbiol. 2015, 15, 56. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, M.N.; Perez, A.A.; Madayag, R.M.; Bottone, E.J. Inhibition of Acanthamoeba species by Pseudomonas aeruginosa: Rationale for their selective exclusion in corneal ulcers and contact lens care systems. J. Clin. Microbiol. 1993, 31, 1908–1910. [Google Scholar] [CrossRef] [Green Version]
- Matz, C.; Moreno, A.M.; Alhede, M.; Manefield, M.; Hauser, A.R.; Givskov, M.; Kjelleberg, S. Pseudomonas aeruginosa uses type III secretion system to kill biofilm-associated amoebae. ISME J. 2008, 2, 843–852. [Google Scholar] [CrossRef]
- El-Sheekh, M.; El-Sabbagh, S.M.; Gharieb, M.M.; Hamza, W.T. Antimicrobial efficacy of pyocyanin produced by Pseudomonas aeruginosa against multi-drug resistant microorganisms. Indian J. Biotechnol. 2014, 9, 498–504. [Google Scholar]
- Kerr, J.R.; Taylor, G.W.; Rutman, A.; Høiby, N.; Cole, P.J.; Wilson, R. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J. Clin. Pathol. 1999, 52, 385–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiewcharoen, S.; Junnu, V.; Chinabut, P. In vitro effect of antifungal drugs on pathogenic Naegleria spp. Southeast Asian J. Trop. Med. Public Health 2002, 33, 38–41. [Google Scholar] [PubMed]
- Clark, L.L.; Dajcs, J.J.; McLean, C.H.; Bartell, J.G.; Stroman, D.W. Pseudomonas otitidis sp. nov., isolated from patients with otic infections. Int. J. Syst. Evol. Microbiol. 2006, 56, 709–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochman, H.; Lerat, E.; Daubin, V. Examining bacterial species under the specter of gene transfer and exchange. Proc. Natl. Acad. Sci. USA 2005, 102, 6595–6599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petti, C.A.; Polage, C.R.; Schreckenberger, P. The role of 16S rRNA gene sequencing in identification of microorganisms misidentified by conventional methods. J. Clin. Microbiol. 2005, 43, 6123–6125. [Google Scholar] [CrossRef] [Green Version]
- Bottone, E.J.; Madayag, R.M.; Qureshi, M.N. Acanthaomeba keratitis: Synergy between amebic and bacterial cocontaminants in cantact lens care systems as a prelude to infection. J. Clin. Microbiol. 1992, 30, 2447–2450. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Thavasi, R.; Jayalakshmi, S. Phenazine pigments from Pseudomonas aeruginosa and their application as antibacterial agent and food colourants. Res. J. Microbiol. 2008, 3, 122–128. [Google Scholar]
- Cárdenas-Zúñiga, R.; Silva-Olivares, A.; Villalba-Magdaleno, J.A.; Sánchez-Monroy, V.; Serrano-Luna, J.; Shibayama, M. Amphotericin B induces apoptosis-like programmed cell death in Naegleria fowleri and Naegleria gruberi. Microbiology 2017, 163, 940–949. [Google Scholar] [CrossRef]
- McDermott, C.; Chess-Williams, R.; Grant, G.D.; Perkins, A.V.; McFarland, A.J.; Davey, A.K.; Anoopkumar-Dukie, S. Effects of Pseudomonas aeruginosa virulence factor pyocyanin on human urothelial cell function and viability. J. Urol. 2012, 187, 1087–1093. [Google Scholar] [CrossRef]
- Lau, G.W.; Ran, H.; Kong, F.; Hassett, D.J.; Mavrodi, D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect. Immun. 2004, 72, 4275–4278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hempenstall, A.; Grant, G.D.; Anoopkumar-Dukie, S.; Johnson, P.J. Pyocyanin inhibits both nitric oxide-dependent and independent relaxation in porcine coronary arteries. Clin. Exp. Pharmacol. Physiol. 2015, 42, 186–191. [Google Scholar] [CrossRef] [PubMed]
- McFarland, A.J.; Grant, G.D.; Perkins, A.V.; Flegg, C.; Davey, A.K.; Allsopp, T.J.; Renshaw, G.; Kavanagh, J.; McDermott, C.M.; Anoopkumar-Dukie, S. Paradoxical role of 3-methyladenine in pyocyanin-induced toxicity in 1321N1 astrocytoma and SH-SY5Y neuroblastoma cells. Int. J. Toxicol. 2013, 32, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Tiewcharoen, S.; Malainual, N.; Junna, V.; Chetanachan, P.; Rabablert, J. Cytopathogenesis of Naegleria fowleri Thai strains for cultured human neuroblastoma cells. Parasitol. Res. 2008, 102, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Postelnicu, T. Probit Analysis. In International Encyclopedia of Statistical Science; Lovric, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1128–1131. [Google Scholar]
- Pang, H.; Tan, Z.; Qin, G.; Wang, Y.; Li, Z.; Cai, Y. Phenotypic and phylogenetic analysis of lactic acid bacteria isolated from forage crops and grasses in the Tibetan Plateau. J. Microbiol. 2012, 50, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]







| Biochemical Tests | KP-01 | KP-14 | KP-15 |
|---|---|---|---|
| Glucose | − | − | + |
| Raffinose | − | − | + |
| Inositol | − | − | + |
| Urease | − | − | − |
| Lysine decarboxylase | − | − | − |
| Tryptophan deaminase activity (TDA) | − | − | − |
| Citrate | + | + | + |
| 1,2,4,5-Tetrachlorobenzene (CL4) | − | − | + |
| Acetate | + | − | − |
| Sucrose | − | − | + |
| Rhamnose | − | − | − |
| Hydrogen sulfide (H2S) | − | − | − |
| Arginine dihydrolase | + | + | − |
| Esculin | − | − | + |
| Maltose | + | + | + |
| Nitrate | + | − | + |
| Sorbital | − | − | + |
| Arabinose | − | − | − |
| Indole | − | − | − |
| Ornithine decarboxylase | − | − | + |
| Voges–Proskauer | − | − | + |
| Ortho-nitrophenyl-β-D-galactopyranoside (ONPG) | − | − | + |
| Oxidase | + | + | − |
| Oxidative fermentation (OF)/glucose | + | + | + |
| Genus and Species Identification | Pseudomonas aeruginosa | Pseudomonas putida | Enterobacter cloacae |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruenchit, P.; Whangviboonkij, N.; Sawasdipokin, H.; Phumisantiphong, U.; Chaicumpa, W. A Search for Anti-Naegleria fowleri Agents Based on Competitive Exclusion Behavior of Microorganisms in Natural Aquatic Environments. Pathogens 2021, 10, 142. https://doi.org/10.3390/pathogens10020142
Ruenchit P, Whangviboonkij N, Sawasdipokin H, Phumisantiphong U, Chaicumpa W. A Search for Anti-Naegleria fowleri Agents Based on Competitive Exclusion Behavior of Microorganisms in Natural Aquatic Environments. Pathogens. 2021; 10(2):142. https://doi.org/10.3390/pathogens10020142
Chicago/Turabian StyleRuenchit, Pichet, Narisara Whangviboonkij, Hathai Sawasdipokin, Uraporn Phumisantiphong, and Wanpen Chaicumpa. 2021. "A Search for Anti-Naegleria fowleri Agents Based on Competitive Exclusion Behavior of Microorganisms in Natural Aquatic Environments" Pathogens 10, no. 2: 142. https://doi.org/10.3390/pathogens10020142