Bioactivity of Carrageenans in Metabolic Syndrome and Cardiovascular Diseases
Abstract
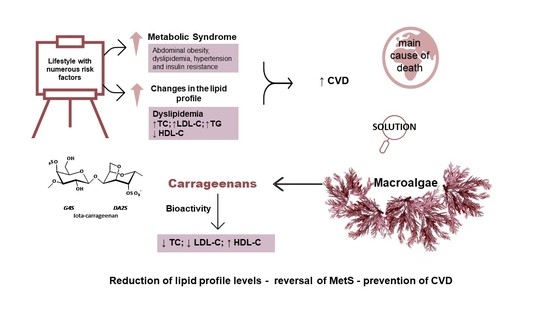
:1. Introduction
2. Metabolic Syndrome
2.1. Lipid Profile and Metabolism
2.2. Cardiovascular Diseases
3. Carrageenans
3.1. Carrageenans for Human Consumption
Carrageenans in the Food Industry: Concept and Future Road
3.2. Carrageenans in Human Health
4. The Potential of the Carrageenans in the Prevention of MetS
4.1. The Potential of Carrageenans in the Prevention of CVD
4.2. Studies Carried Out in Animal Models
4.3. Studies Carried Out in Humans
5. Current and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakurai, R.; Zuchi, J.D. Industrial revolutions to industry 4.0. Interface Tecnol. 2018, 15, 480–491. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Mirosa, M.; Bremer, P. Review of Online Food Delivery Platforms and their Impacts on Sustainability. Sustainability 2020, 12, 5528. [Google Scholar] [CrossRef]
- Park, J.H.; Moon, J.H.; Kim, H.J.; Kong, M.H.; Oh, Y.H. Sedentary Lifestyle: Overview of Updated Evidence of Potential Health Risks. Korean J. Fam. Med. 2020, 41, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Kopčeková, J.; Holovičová, M.; Gažarová, M.; Mrázová, J.; Habánová, M.; Mečiarová, L.; Bronkowska, M. Association between Selected Dietary Habits and Lipid Profiles of Patients with Cardiovascular Disease. Int. J. Environ. Res. Public Health 2020, 17, 7605. [Google Scholar] [CrossRef]
- Cortez-Dias, N.; Robalo Martins, S.; Belo, A.; Fiúza, M. Characterization of lipid profile in primary health care users in Portugal. Rev. Port. Cardiol. 2013, 32, 987–996. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Guembe, M.J.; Fernandez-Lazaro, C.I.; Sayon-Orea, C.; Toledo, E.; Moreno-Iribas, C.; Investigators, R.S. Risk for cardiovascular disease associated with metabolic syndrome and its components: A 13-year prospective study in the RIVANA cohort. Cardiovasc. Diabetol. 2020, 19, 195. [Google Scholar] [CrossRef]
- Li, X.; Zhai, Y.; Zhao, J.; He, H.; Li, Y.; Liu, Y.; Feng, A.; Li, L.; Huang, T.; Xu, A.; et al. Impact of Metabolic Syndrome and It’s Components on Prognosis in Patients With Cardiovascular Diseases: A Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 704145. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- WHO—World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 25 October 2022).
- Kruk, M.E.; Gage, A.D.; Arsenault, C.; Jordan, K.; Leslie, H.H.; Roder-DeWan, S.; Adeyi, O.; Barker, P.; Daelmans, B.; Doubova, S.V.; et al. High-quality health systems in the Sustainable Development Goals era: Time for a revolution. Lancet Glob. Health 2018, 6, e1196–e1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuckler, D. Population causes and consequences of leading chronic diseases: A comparative analysis of prevailing explanations. Milbank Q. 2008, 86, 273–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nystrand, B.T.; Olsen, S.O. Consumers’ attitudes and intentions toward consuming functional foods in Norway. Food Qual. Prefer. 2020, 80, 103827. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.; Pinto, D.C.; Silva, A.M. Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods. Mar. Drugs 2015, 13, 6838. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L. Biological and therapeutic properties of the seaweed polysaccharides. Int. Biol. Rev. 2018, 2, 1762. [Google Scholar] [CrossRef] [Green Version]
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Solanki, H.K. Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014, 105, 97–112. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Bogdanovich, L.N.; Ivanova, T.B.; Byankina, A.O.; Kryzhanovskiy, S.P.; Yermak, I.M. Effect of carrageenan food supplement on patients with cardiovascular disease results in normalization of lipid profile and moderate modulation of immunity system markers. PharmaNutrition 2014, 2, 33–37. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Feferman, L.; Unterman, T.; Tobacman, J.K. Exposure to Common Food Additive Carrageenan Alone Leads to Fasting Hyperglycemia and in Combination with High Fat Diet Exacerbates Glucose Intolerance and Hyperlipidemia without Effect on Weight. J. Diabetes Res. 2015, 2015, 513429. [Google Scholar] [CrossRef]
- du Preez, R.; Paul, N.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Carrageenans from the red seaweed Sarconema filiforme attenuate symptoms of diet-Induced metabolic syndrome in rats. Mar. Drugs 2020, 18, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Chávez, A.; Chávez-Fernández, J.A.; Elizondo-Argueta, S.; González-Tapia, A.; León-Pedroza, J.I.; Ochoa, C. Metabolic Syndrome and Cardiovascular Disease: A Health Challenge. Arch. Med. Res. 2018, 49, 516–521. [Google Scholar] [CrossRef] [PubMed]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome: A multiplex cardiovascular risk factor. J. Clin. Endocrinol. Metab. 2007, 92, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.P.; Williams, K.; González-Villalpando, C.; Hunt, K.J.; Haffner, S.M. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care 2004, 27, 2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Qin, J.; Cheng, Y.; Lv, D.; Li, M.; Qi, Y.; Lan, J.; Zhao, Q.; Li, Z. Marine Sulfated Polysaccharides: Preventive and Therapeutic Effects on Metabolic Syndrome: A Review. Mar. Drugs 2021, 19, 608. [Google Scholar] [CrossRef]
- Solnica, B.; Sygitowicz, G.; Sitkiewicz, D.; Cybulska, B.; Jóźwiak, J.; Odrowąż-Sypniewska, G.; Banach, M. 2020 Guidelines of the Polish Society of Laboratory Diagnostics (PSLD) and the Polish Lipid Association (PoLA) on laboratory diagnostics of lipid metabolism disorders. Arch. Med. Sci. 2020, 16, 237–252. [Google Scholar] [CrossRef]
- Zárate, A.; Manuel-Apolinar, L.; Basurto, L.; De la Chesnaye, E.; Saldívar, I. Cholesterol and atherosclerosis. Historical considerations and treatment. Arch. Cardiol. Mex. 2016, 86, 163–169. [Google Scholar] [CrossRef]
- Hall, J.E.; Hall, M.E.; Guyton, A.C. Textbook of Medical Physiology, 14th ed.; Elsevier: Amsterdam, The Netherlands, 2021; p. 1152. ISBN 9780323597128. [Google Scholar]
- Dousip, A.; Matanjun, P.; Sulaiman, M.R.; Tan, T.S.; Ooi, Y.B.H.; Lim, T.P. Effect of seaweed mixture intake on plasma lipid and antioxidant profile of hyperholesterolaemic rats. J. Appl. Phycol. 2013, 26, 999–1008. [Google Scholar] [CrossRef]
- Pereira, L.; Soares, F.; Freitas, A.C.; Duarte, A.C.; Ribeiro-Claro, P. Extraction, Characterization, and Use of Carrageenans (Chapter 3). In Industrial Applications of Marine Biopolymers; Sudha, P.N., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2017; pp. 34–89, 626. ISBN 9781498731485. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L. Carrageenans: Sources and Extraction Methods, Molecular Structure, Bioactive Properties and Health Effects; Nova Science Publishers: New York, NY, USA, 2016; p. 304. ISBN 978-1-63485-503-7. [Google Scholar]
- Pereira, L. Vibrational Spectroscopy of Seaweed Polysaccharides. In Seaweed Polysaccharides—Isolation, Biological and Biomedical Applications; Venkatesan, J., Anil, S., Kim, S.-K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 83–100. ISBN 978-0-12-809816-5. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. In Advances in Carbohydrate Chemistry and Biochemistry; Horton, D., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 115–217. [Google Scholar] [CrossRef]
- Pereira, L.; van de Velde, F. Portuguese carrageenophytes: Carrageenan composition and geographic distribution of eight species (Gigartinales, Rhodophyta). Carbohydr. Polym. 2011, 84, 614–623. [Google Scholar] [CrossRef] [Green Version]
- Tanusorn, N.; Thummarungsan, N.; Sangwan, W.; Lerdwijitjarud, W.; Sirivat, A. Influence of carrageenan molecular structures on electromechanical behaviours of poly(3-hexylthiophene)/carrageenan conductive hydrogels. Int. J. Biol. Macromol. 2018, 118, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U. Re-evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives. EFSA J. 2018, 16, e05238. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CREU—Commission Regulation (EU) No 1129/2011 of 11 November 2011. Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by Establishing a Union list of Food Additives Text with EEA Relevance. Available online: http://data.europa.eu/eli/reg/2011/1129/oj (accessed on 25 October 2022).
- McKim, J.M.; Baas, H.; Rice, G.P.; Willoughby, J.A.; Weiner, M.L.; Blakemore, W. Effects of carrageenan on cell permeability, cytotoxicity, and cytokine gene expression in human intestinal and hepatic cell lines. Food Chem. Toxicol. 2016, 96, 1–10. [Google Scholar] [CrossRef]
- Cohen, S.M.; Ito, N. A Critical Review of the Toxicological Effects of Carrageenan and Processed Eucheuma Seaweed on the Gastrointestinal Tract. Crit. Rev. Toxicol. 2002, 32, 413–444. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Evaluation of Certain Food Additives and Contaminants: Eightieth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2016; Available online: https://apps.who.int/iris/handle/10665/204410 (accessed on 25 October 2022).
- Weiner, M.L. Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies. Crit. Rev. Toxicol. 2014, 44, 244–269. [Google Scholar] [CrossRef]
- FDA—US Food & Drug Administration. CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=172.620 (accessed on 25 October 2022).
- Bhattacharyya, S.; Borthakur, A.; Dudeja, P.K.; Tobacman, J.K. Carrageenan Reduces Bone Morphogenetic Protein-4 (BMP4) and Activates the Wnt/β-Catenin Pathway in Normal Human Colonocytes. Dig. Dis. Sci. 2007, 52, 2766–2774. [Google Scholar] [CrossRef]
- Tobacman, J.K. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ. Health Perspect. 2001, 109, 983–994. [Google Scholar] [CrossRef]
- Tobacman, J.K. Filament disassembly and loss of mammary myoepithelial cells after exposure to lambda-carrageenan. Cancer Res. 1997, 57, 2823–2826. [Google Scholar] [PubMed]
- Weiner, M.L.; McKim, J.M.; Blakemore, W.R. Addendum to Weiner, M.L. (2016) Parameters and Pitfalls to Consider in the Conduct of Food Additive Research, Carrageenan as a Case Study. Food Chemical Toxicology 87, 31–44. Food Chem. Toxicol. 2017, 107, 208–214. [Google Scholar] [CrossRef]
- Blakemore, W.R.; Davis, S.R.; Hroncich, M.M.; Vurma, M. Carrageenan analysis. Part 1: Characterisation of the carrageenan test material and stability in swine-adapted infant formula. Food Addit. Contam. 2014, 31, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, W.R.; Brant, A.F.; Bissland, J.G.; Bissland, N.D. Carrageenan analysis. Part 3: Quantification in swine plasma. Food Addit. Contam. 2014, 31, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Borsani, B.; De Santis, R.; Perico, V.; Penagini, F.; Pendezza, E.; Dilillo, D.; Bosetti, A.; Zuccotti, G.V.; D’Auria, E. The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand? Nutrients 2021, 13, 3402. [Google Scholar] [CrossRef]
- McKim, J.M. Food additive carrageenan: Part I: A critical review of carrageenan in vitro studies, potential pitfalls, and implications for human health and safety. Crit. Rev. Toxicol. 2014, 44, 211–243. [Google Scholar] [CrossRef]
- Hotchkiss, S.; Brooks, M.; Campbell, R.; Philp, K.; Trius, A. The use of carrageenan in food. In Carrageenans: Sources and Extraction Methods, Molecular Structure, Bioactive Properties and Health Effects; Pereira, L., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 229–243. ISBN 978-1-63485-503-7. [Google Scholar]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Shimazu, T.; Kuriyama, S.; Hozawa, A.; Ohmori, K.; Sato, Y.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Dietary patterns and cardiovascular disease mortality in Japan: A prospective cohort study. Int. J. Epidemiol. 2007, 36, 600–609. [Google Scholar] [CrossRef] [Green Version]
- Yamori, Y.; Miura, A.; Taira, K. Implications from and for food cultures for cardiovascular diseases: Japanese food, particularly Okinawan diets. Asia Pac. J. Clin. Nutr. 2001, 10, 144–145. [Google Scholar] [CrossRef]
- Zaporozhets, T.; Besednova, N. Prospects for the therapeutic application of sulfated polysaccharides of brown algae in diseases of the cardiovascular system: Review. Pharm. Biol. 2016, 54, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; p. 654. ISBN 9780702050329. [Google Scholar]
- Capuano, E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amano, H.; Kakinuma, M.; Coury, D.A.; Ohno, H.; Hara, T. Effect of a seaweed mixture on serum lipid level and platelet aggregation in rats. Fish. Sci. 2005, 71, 1160–1166. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Singh, H. Biophysical insights into modulating lipid digestion in food emulsions. Prog. Lipid Res. 2022, 85, 101129. [Google Scholar] [CrossRef]
- Panlasigui, L.N.; Baello, O.Q.; Dimatangal, J.M.; Dumelod, B.D. Blood cholesterol and lipid-lowering effects of carrageenan on human volunteers. Asia Pac. J. Clin. Nutr. 2003, 12, 209–214. [Google Scholar]
- Chater, P.I.; Wilcox, M.; Cherry, P.; Herford, A.; Mustar, S.; Wheater, H.; Brownlee, I.; Seal, C.; Pearson, J. Inhibitory activity of extracts of Hebridean brown seaweeds on lipase activity. J. Appl. Phycol. 2016, 28, 1303–1313. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Onnagawa, M.; Yoshie, Y.; Suzuki, T. Binding of bile salts to soluble and insoluble dietary fibers of seaweeds. Fish. Sci. 2001, 67, 1169–1173. [Google Scholar] [CrossRef]
- Matthan, N.R.; Zhu, L.; Pencina, M.; D’Agostino, R.B.; Schaefer, E.J.; Lichtenstein, A.H. Sex-specific differences in the predictive value of cholesterol homeostasis markers and 10-year cardiovascular disease event rate in Framingham Offspring Study participants. J. Am. Heart Assoc. 2013, 2, e005066. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, M.; Miller, C. A diet containing food rich in soluble and insoluble fiber improves glycemic control and reduces hyperlipidemia among patients with type 2 diabetes mellitus. Nutr. Rev. 2001, 59, 52–55. [Google Scholar] [CrossRef]
- Wanyonyi, S.; du Preez, R.; Brown, L.; Paul, N.A.; Panchal, S.K. Kappaphycus alvarezii as a Food Supplement Prevents Diet-Induced Metabolic Syndrome in Rats. Nutrients 2017, 9, 1261. [Google Scholar] [CrossRef] [Green Version]
- Chin, Y.X.; Mi, Y.; Cao, W.X.; Lim, P.E.; Xue, C.H.; Tang, Q.J. A pilot study on anti-obesity mechanisms of Kappaphycus alvarezii: The role of native kappa-carrageenan and the leftover sans-carrageenan Fraction. Nutrients 2019, 11, 1133. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Ordonez, E.; Jimenez-Escrig, A.; Ruperez, P. Effect of the red seaweed Mastocarpus stellatus intake on lipid metabolism and antioxidant status in healthy Wistar rats. Food Chem. 2012, 135, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Hasanuddin, A.; Rusdi; Arief, R. Effects of inclusion of fermented carrageenan by-products in the basal diet of broiler chickens on growth performance, blood profiles and meat composition. Int. J. Poult. Sci. 2017, 16, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Zhong, W. Antihyperglycemic and antihyperlipidemic effects of low-molecular-weight carrageenan in rats. Open Life Sci. 2018, 13, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.C.; Elias, J.; Kelley, J.J.; Lin, R.S.; Robson, J.R. Influence of certain dietary fibers on serum and tissue cholesterol levels in rats. J. Nutr. 1976, 106, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Hasanuddinrusdi, A. Evaluation of nutritional values of fermented carrageenan by-product of seaweed (Eucheuma cottonii) raw materials. Agrisains 2012, 13, 159–166. [Google Scholar]
- Valado, A.; Pereira, M.; Caseiro, A.; Figueiredo, J.P.; Loureiro, H.; Almeida, C.; Cotas, J.; Pereira, L. Effect of Carrageenans on Vegetable Jelly in Humans with Hypercholesterolemia. Mar. Drugs 2019, 18, 19. [Google Scholar] [CrossRef]

| Authors | Model | Period | Carrageenans % | Mode | MetS | Lipid Profile |
|---|---|---|---|---|---|---|
| Hasanuddin et al. [73] | chicks | 5 weeks | 2.5 fermented CC | Diet | - | ↓TC *; ↓LDL-C *; ↑HDL-C *; ↓TG * |
| 5 fermented CC | - | ↓TC *; ↓LDL-C *; ↑HDL-C *; ↓TG * | ||||
| 7.5 fermented CC | - | ↓TC *; ↓LDL-C *; ↑HDL-C *; ↓TG * | ||||
| 10 fermented CC | - | ↓TC *; ↓LDL-C *; ↑HDL-C *; ↓TG * | ||||
| Wanyonyi et al. [70] | rats | 8 weeks | HFD + 5 K. alvarezii | Diet | ↓BW; ↓F; ↓AC; ↓SBP | ↑TC; ↓TG |
| Xia et al. [74] | rats | 30 days | HC + 1 CC | Diet | ↓BW | ↓TC; ↓LDL-C; ↑HDL-C; ↓TG |
| HC + 1 LC | ↓BW | ↓TC *; ↓LDL-C; ↑HDL-C; ↓TG * | ||||
| HC + 3 LC | ↓BW | ↓TC *; ↓LDL-C *; ↑HDL-C; ↓TG * | ||||
| Du Preez et al. [22] | rats | 16 weeks | HFD + 5 S. filiforme | Diet | ↓BW; ↓F; ↓AC; ↓SBP | ↓TC *; ↓TG * |
| Corn Starch + 5 S. filiforme | ↓BW; ↓F; ↓AC; ↓SBP | ↓TC *; ↓TG * | ||||
| Bhattacharyya et al. [21] | mice | 44 weeks | CGN | Water | ↑BW; | ↑TC; ↑LDL-C; ↓HDL-C; ↑TG |
| HFD + CGN | ↑BW; | ↑TC *; ↑LDL-C *; ↑HDL-C *; ↑TG * | ||||
| Chin et al. [71] | mice | 16 weeks | HFD + 5 K. alvarezii | Diet | ↓BW; ↓F | ↓TC; ↓LDL-C; ↑HDL-C; ↓TG |
| HFD + 5 k-CGN | ↓BW *; ↓F | ↓TC; ↓LDL-C; ↑HDL-C; ↓TG | ||||
| HFD + 5 SCCGN | ↓BW *; ↓F * | ↓TC; ↓LDL-C; ↑HDL-C *; ↓TG | ||||
| Tsai et al. [75] | rats | 32 days | 7 CC | Diet | - | ↑TC |
| 42 days | - | ↓TC | ||||
| Gómez-Ordóñez et al. [72] | rats | 4 weeks | 10 Mastocarpus stellatus | Diet | - | ↓TC *; ↓TG * |
| Panlasigui et al. [65] | humans | 8 weeks | 40 g/day, in food | Diet | - | ↓TC *; ↓TG *; ↑LDL-C; ↑HDL-C * |
| Sokolova et al. [20] | humans | 20 weeks | Capsules 250 mg | Capsule | - | ↓TC *; ↑TG *; ↓LDL-C *; ↑HDL-C |
| Valado et al. [77] | humans | 30 days | 100 mL/day of vegetable jelly | Diet | - | ↓TC *; ↑TG *; ↓LDL-C; ↓HDL-C * |
| 60 days | - | ↓TC *; ↑TG *; ↓LDL-C; ↓HDL-C * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valado, A.; Pereira, M.; Amaral, M.; Cotas, J.; Pereira, L. Bioactivity of Carrageenans in Metabolic Syndrome and Cardiovascular Diseases. Nutraceuticals 2022, 2, 441-454. https://doi.org/10.3390/nutraceuticals2040032
Valado A, Pereira M, Amaral M, Cotas J, Pereira L. Bioactivity of Carrageenans in Metabolic Syndrome and Cardiovascular Diseases. Nutraceuticals. 2022; 2(4):441-454. https://doi.org/10.3390/nutraceuticals2040032
Chicago/Turabian StyleValado, Ana, Maria Pereira, Mónica Amaral, João Cotas, and Leonel Pereira. 2022. "Bioactivity of Carrageenans in Metabolic Syndrome and Cardiovascular Diseases" Nutraceuticals 2, no. 4: 441-454. https://doi.org/10.3390/nutraceuticals2040032