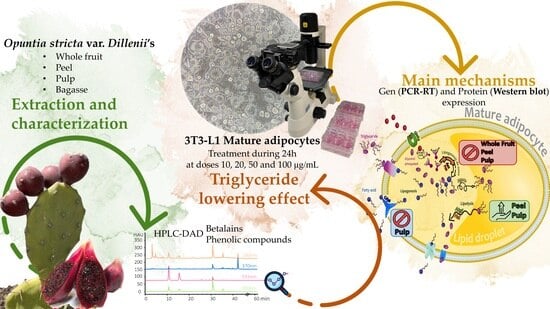
Anti-Obesity Effect of Different Opuntia stricta var. dillenii’s Prickly Pear Tissues and Industrial By-Product Extracts in 3T3-L1 Mature Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Opuntia stricta var. dillenii Extracts
2.3. Characterisation of Opuntia Extracts
2.3.1. HPLC Analysis of Betalain and Phenolic Compounds
2.3.2. Determination of the Chemical In Vitro Antioxidant Activity Using ORAC Method and LOX-FL
2.4. Mature Adipocyte Cell Experimental Design
2.4.1. Cell Treatment
2.4.2. Cell Viability Assay
2.4.3. Measurement of Triglyceride and Protein Content in 3T3-L1 Mature Adipocytes
2.4.4. RNA Extraction and RT-PCR
2.4.5. Western Blot Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Opuntia stricta var. dillenii Extracts Characterisation and In Vitro Biological Activities
3.1.1. Bioactive Compounds from Opuntia stricta var. dillenii Extracts: Betalains and Phenolic Compounds
Betalains
Phenolic Compounds
3.1.2. In Vitro Biological Activities of Opuntia stricta var. dillenii Extracts
3.2. Effects of Opuntia stricta var. dillenii Extracts in 3T3-L1 Mature Adipocytes
3.2.1. Effects on Cell Viability
3.2.2. Effects on Triglyceride Content
3.2.3. Effects on Genes and Proteins Involved in 3T3-L1 Mature Adipocytes Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 18 September 2023).
- WHO. Noncommunicable Diseases Progress Monitor 2022. 2022. Available online: https://www.who.int/publications-detail-redirect/9789240047761 (accessed on 18 September 2023).
- Di Mauro, M.; Taylor, V.; Wharton, S.; Sharma, A.M. Barriers to Obesity Treatment. Eur. J. Intern. Med. 2008, 19, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Bleich, S.N.; Jones-Smith, J.; Wolfson, J.A.; Zhu, X.; Story, M. The Complex Relationship between Diet and Health. Health Aff. 2015, 34, 1813–1820. [Google Scholar] [CrossRef]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological Effects of Red Beetroot and Betalains: A Review. Phytother. Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef] [PubMed]
- González-Arceo, M.; Gomez-Lopez, I.; Carr-Ugarte, H.; Eseberri, I.; González, M.; Cano, M.P.; Portillo, M.P.; Gómez-Zorita, S. Anti-Obesity Effects of Isorhamnetin and Isorhamnetin Conjugates. Int. J. Mol. Sci. 2022, 24, 299. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Asante-Donyinah, D.; Letsyo, E.; Dzikunoo, J.; Adams, Z.S. Dietary Polyphenols and Obesity: A Review of Polyphenol Effects on Lipid and Glucose Metabolism, Mitochondrial Homeostasis, and Starch Digestibility and Absorption. Plant Foods Hum. Nutr. 2023, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Di Bona, D.; Candore, G.; Carru, C.; Zinellu, A.; Di Miceli, G.; Nicosia, A.; Gambino, C.M.; Ruisi, P.; Caruso, C.; et al. Targeting Aging with Functional Food: Pasta with Opuntia Single-Arm Pilot Study. Rejuvenation Res. 2018, 21, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. In Silico Investigation on the Interaction of Chiral Phytochemicals from Opuntia ficus-indica with SARS-CoV-2 Mpro. Symmetry 2021, 13, 1041. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Portillo-Reyes, J.; Madrigal-Bujaidar, E.; Sánchez-Gutiérrez, M.; Mercado-Gonzalez, P.; Izquierdo-Vega, J.; Vargas-Mendoza, N.; Álvarez-González, I.; Fregoso-Aguilar, T.; Delgado-Olivares, L.; et al. Opuntia Genus in Human Health: A Comprehensive Summary on its Pharmacological, Therapeutic and Preventive Properties. Part 1. Horticulturae 2022, 8, 88. [Google Scholar] [CrossRef]
- Attanzio, A.; Restivo, I.; Tutone, M.; Tesoriere, L.; Allegra, M.; Livrea, M.A. Redox Properties, Bioactivity and Health Effects of Indicaxanthin, a Bioavailable Phytochemical from Opuntia ficus indica, L.: A Critical Review of Accumulated Evidence and Perspectives. Antioxidants 2022, 11, 2364. [Google Scholar] [CrossRef]
- Kour, J.; Nayik, G.A. Nutraceuticals and Health Care; Academic Press: San Diego, CA, USA, 2021; pp. 87–104. [Google Scholar]
- Shoukat, R.; Cappai, M.; Pia, G.; Pilia, L. An Updated Review: Opuntia ficus indica (OFI) Chemistry and its Diverse Applications. Appl. Sci. 2023, 13, 7724. [Google Scholar] [CrossRef]
- Pérez-Molphe-Balch, E.; Santos-Díaz, M.d.S.; Ramírez-Malagón, R.; Ochoa-Alejo, N. Tissue Culture of Ornamental Cacti. Sci. Agric. 2015, 72, 540–561. [Google Scholar] [CrossRef]
- Mondragón-Jacobo, C.; Pérez-González, S. Cactus (Opuntia spp.) as Forage. 2001. Available online: https://www.fao.org/3/y2808e/y2808e.pdf (accessed on 21 September 2023).
- Shirazinia, R.; Rahimi, V.B.; Kehkhaie, A.R.; Sahebkar, A.; Rakhshandeh, H.; Askari, V.R. Opuntia DilleniI: A Forgotten Plant with Promising Pharmacological Properties. J. Pharmacopunct. 2019, 22, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, I.; Lobo-Rodrigo, G.; Portillo, M.P.; Cano, M.P. Characterization, Stability, and Bioaccessibility of Betalain and Phenolic Compounds from Opuntia stricta var. Dillenii Fruits and Products of their Industrialization. Foods 2021, 10, 1593. [Google Scholar] [CrossRef]
- Thi Tran, T.M.; Nguyen Thanh, B.; Moussa-Ayoub, T.E.; Rohn, S.; Jerz, G. Profiling of Polar Metabolites in Fruits of Opuntia stricta var. Dillenii by Ion-Pair High-Performance Countercurrent Chromatography and Off-Line Electrospray Mass-Spectrometry Injection. J. Chromatogr. A 2019, 1601, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Medina, E.M.; Rodríguez, E.M.R.; Romero, C.D. Chemical Characterization of Opuntia dillenii and Opuntia ficus indica Fruits. Food Chem. 2007, 103, 38–45. [Google Scholar] [CrossRef]
- Betancourt, C.; Cejudo-Bastante, M.J.; Heredia, F.J.; Hurtado, N. Pigment Composition and Antioxidant Capacity of Betacyanins and Betaxanthins Fractions of Opuntia dillenii (Ker Gawl) Haw Cactus Fruit. Food Res. Int. 2017, 101, 173–179. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional Properties of Anthocyanins and Betalains in Plants, Food, and in Human Nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Song, H.; Chu, Q.; Xu, D.; Xu, Y.; Zheng, X. Purified Betacyanins from Hylocereus Undatus Peel Ameliorate Obesity and Insulin Resistance in High-Fat-Diet-Fed Mice. J. Agric. Food Chem. 2016, 64, 236–244. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Martínez-Vitela, C.; Serna-Saldívar, S.O. Topical Anti-Inflammatory Effects of Isorhamnetin Glycosides Isolated from Opuntia ficus-indica. BioMed Res. Int. 2015, 2015, 847320. [Google Scholar] [CrossRef]
- Zeghbib, W.; Boudjouan, F.; Vasconcelos, V.; Lopes, G. Phenolic Compounds’ Occurrence in Opuntia Species and their Role in the Inflammatory Process: A Review. Molecules 2022, 27, 4763. [Google Scholar] [CrossRef] [PubMed]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and Quantification of Individual Betalain and Phenolic Compounds in Mexican and Spanish Prickly Pear (Opuntia Ficus-Indica L. Mill) Tissues: A Comparative Study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Aleixandre-Tudó, J.L.; du Toit, W. The Role of UV-Visible Spectroscopy for Phenolic Compounds Quantification in Winemaking. In Frontiers and New Trends in the Science of Fermented Food and Beverages; IntechOpen: London, UK, 2019. [Google Scholar]
- Zhang, D.; Lanier, S.M.; Downing, J.A.; Avent, J.L.; Lum, J.; McHale, J.L. Betalain Pigments for Dye-Sensitized Solar Cells. J. Photochem. Photobiol. A Chem. 2008, 195, 72–80. [Google Scholar] [CrossRef]
- Montiel-Sánchez, M.; García-Cayuela, T.; Gómez-Maqueo, A.; García, H.S.; Cano, M.P. In Vitro Gastrointestinal Stability, Bioaccessibility and Potential Biological Activities of Betalains and Phenolic Compounds in Cactus Berry Fruits (Myrtillocactus geometrizans). Food Chem. 2021, 342, 128087. [Google Scholar] [CrossRef]
- Gómez-López, I.; Mendiola, J.A.; Portillo, M.P.; Cano, M.P. Pressurized Green Liquid Extraction of Betalains and Phenolic Compounds from Opuntia stricta var. Dillenii Whole Fruit: Process Optimization and Biological Activities of Green Extracts. Innov. Food Sci. Emerg. Technol. 2022, 80, 103066. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Soccio, M.; Cano, M.P. In Vitro Antioxidant Capacity of Opuntia Spp. Fruits Measured by the LOX-FL Method and its High Sensitivity towards Betalains. Plant Foods Hum. Nutr. 2021, 76, 354–362. [Google Scholar] [CrossRef]
- Gillies, R.J.; Didier, N.; Denton, M. Determination of Cell Number in Monolayer Cultures. Anal. Biochem. 1986, 159, 109–113. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Maqueo, A.; Welti-Chanes, J.; Cano, M.P. Release Mechanisms of Bioactive Compounds in Fruits Submitted to High Hydrostatic Pressure: A Dynamic Microstructural Analysis Based on Prickly Pear Cells. Food Res. Int. 2020, 130, 108909. [Google Scholar] [CrossRef] [PubMed]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Soccio, M.; Laus, M.N.; Flagella, Z.; Pastore, D. Assessment of Antioxidant Capacity and Putative Healthy Effects of Natural Plant Products using Soybean Lipoxygenase-Based Methods. An Overview. Molecules 2018, 23, 3244. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Abderrabba, M. Chemical and Antioxidant Properties of Betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Fernando, G.S.N.; Sergeeva, N.N.; Frutos, M.J.; Marshall, L.J.; Boesch, C. Novel Approach for Purification of Major Betalains using Flash Chromatography and Comparison of Radical Scavenging and Antioxidant Activities. Food Chem. 2022, 385, 132632. [Google Scholar] [CrossRef]
- Del Socorro Santos Díaz, M.; Barba de la Rosa, A.P.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntia Spp.: Characterization and Benefits in Chronic Diseases. Oxidative Med. Cell. Longev. 2017, 2017, 8634249. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; García-Cayuela, T.; Welti-Chanes, J.; Cano, M.P. Enhancement of Anti-Inflammatory and Antioxidant Activities of Prickly Pear Fruits by High Hydrostatic Pressure: A Chemical and Microstructural Approach. Innov. Food Sci. Emerg. Technol. 2019, 54, 132–142. [Google Scholar] [CrossRef]
- Boudjouan, F.; Zeghbib, W.; Carneiro, J.; Silva, R.; Morais, J.; Vasconcelos, V.; Lopes, G. Comparison Study on Wild and Cultivated Opuntia Sp.: Chemical, Taxonomic, and Antioxidant Evaluations. Agriculture 2022, 12, 1755. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; He, X.; Tang, Y.; Li, Z.; Li, C.; Zeng, Y.; Tang, J.; Sun, J. Betacyanins and Anthocyanins in Pulp and Peel of Red Pitaya (Hylocereus polyrhizus Cv. Jindu), Inhibition of Oxidative Stress, Lipid Reducing, and Cytotoxic Effects. Front. Nutr. 2022, 9, 894438. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Duncan, R.E.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Triacylglycerol Metabolism in Adipose Tissue. Future Lipidol. 2007, 2, 229–237. [Google Scholar] [CrossRef]







| SYBR Green RT-PCR | |||
|---|---|---|---|
| Gene | Sense Primer | Antisense Primer | Annealing (°C) |
| fas | 5′-AGC CCC TCA AGT GCA CAG T-3′ | 5′-TGC CAA TGT GTT TTC CCT G-3′ | 62.7 |
| cd36 | 5′-GAT GAC GTG GCA AAG AAC AG-3′ | 5′-CAG TGA AGG CTC AAA GAT GG-3′ | 60.7 |
| lpl | 5′-CCT CTC TCC AGG GGA CAA GT-3′ | 5′-GAA GGC GGT CAA CTC TGG A-3′ | 60.0 |
| atgl | 5′-GAG CTT CGC GTC ACC ACC-3′ | 5′-CAC ATC TCT CGG AGG ACC A-3′ | 58.5 |
| Taqman RT-PCR | |||
| Gene | Assay ID | ||
| acc | Mm01304285_m1 | ||
| dgat2 | Mm00499536_m1 | ||
| hls | Mm00495359_m1 | ||
| O. stricta var. dillenii | ||||||
|---|---|---|---|---|---|---|
| Peak * | Compounds | Family | Whole Fruit | Peel | Pulp | Bagasse |
| mg/g Dry Weight | ||||||
| 1 | Piscidic acid | Phenolic acid | 1.64 ± 0.09 b | 2.33 ± 0.33 a | 0.62 ± 0.05 c | 1.54 ± 0.05 b |
| 2 | Betanin | Betalain | 2.97 ± 0.01 a | 2.99 ± 0.05 a | 2.91 ± 0.23 a | 0.84 ± 0.02 b |
| 3 | Isobetanin | Betalain | 1.85 ± 0.00 b | 1.65 ± 0.04 b | 2.28 ± 0.19 a | 0.77 ± 0.02 c |
| 4 | Betanidin | Betalain | 0.04 ± 0.00 a | 0.04 ± 0.00 a | 0.04 ± 0.01 a | 0.02 ± 0.00 b |
| 5 | 6′-O-sinapoyl-O-gompherin | Betalain | 0.13 ± 0.00 b | 0.14 ± 0.00 a | 0.08 ± 0.00 c | 0.01 ± 0.00 d |
| 6 | 2′-O-apiosyl-4-O-phyllocactin | Betalain | 1.29 ± 0.02 a | 1.22 ± 0.02 a | 1.61 ± 0.18 a | 0.58 ± 0.16 b |
| 7 | 5″-O-E-sinapoyl-2′-apyosil-phyllocactin | Betalain | 3.14 ± 0.00 a | 3.23 ± 0.13 a | 2.60 ± 0.07 a | n.d. |
| 8 | Neobetanin | Betalain | 1.95 ± 0.02 b | 0.82 ± 0.00 d | 3.26 ± 0.05 a | 1.03 ± 0.07 c |
| 9 | Quercetin-3-O-rhamnosyl-rutinoside (QG3) | Flavonoid | 0.04 ± 0.00 b | 0.07 ± 0.00 a | n.d. | 0.02 ± 0.00 c |
| 10 | Quercetin glycoside(QG1)—Quercetin hexosyl pentosyl rhamnoside | Flavonoid | 0.04 ± 0.00 b | 0.08 ± 0.00 a | n.d. | 0.02 ± 0.00 c |
| 11 | Quercetin glycoside(QG2)—Quercetin hexose pentoside | Flavonoid | 0.02 ± 0.00 a | 0.02 ± 0.00 a | n.d. | n.d. |
| 12 | Isorhamnetin glucosyl-rhamnosyl-rhamnoside(IG1) | Flavonoid | 0.02 ± 0.00 a | 0.03 ± 0.00 a | n.d. | 0.01 ± 0.00 a |
| 13 | Isorhamnetin glucosyl-rhamnosyl-pentoside(IG2) | Flavonoid | 0.29 ± 0.00 b | 0.52 ± 0.02 a | 0.05 ± 0.00 d | 0.18 ± 0.01 c |
| Total major betalains | 11.37 ± 0.02 b | 10.08 ± 0.03 c | 12.78 ± 0.48 a | 3.24 ± 0.27 d | ||
| Total major phenolic compounds (Pisicidic acid and flavonoids) | 2.06 ± 0.09 b | 3.04 ± 0.02 a | 0.67 ± 0.05d | 1.78 ± 0.06 c | ||
| Total major flavonoids | 0.42 ± 0.00 b | 0.72 ± 0.02 a | 0.05 ± 0.00 d | 0.24 ± 0.01 c | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-López, I.; Eseberri, I.; Cano, M.P.; Portillo, M.P. Anti-Obesity Effect of Different Opuntia stricta var. dillenii’s Prickly Pear Tissues and Industrial By-Product Extracts in 3T3-L1 Mature Adipocytes. Nutrients 2024, 16, 499. https://doi.org/10.3390/nu16040499
Gómez-López I, Eseberri I, Cano MP, Portillo MP. Anti-Obesity Effect of Different Opuntia stricta var. dillenii’s Prickly Pear Tissues and Industrial By-Product Extracts in 3T3-L1 Mature Adipocytes. Nutrients. 2024; 16(4):499. https://doi.org/10.3390/nu16040499
Chicago/Turabian StyleGómez-López, Iván, Itziar Eseberri, M. Pilar Cano, and María P. Portillo. 2024. "Anti-Obesity Effect of Different Opuntia stricta var. dillenii’s Prickly Pear Tissues and Industrial By-Product Extracts in 3T3-L1 Mature Adipocytes" Nutrients 16, no. 4: 499. https://doi.org/10.3390/nu16040499