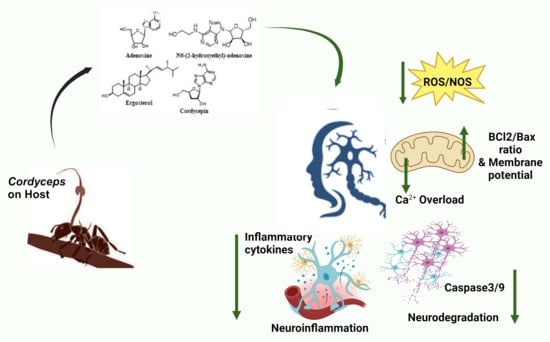
Unique Bioactives from Zombie Fungus (Cordyceps) as Promising Multitargeted Neuroprotective Agents
Abstract
:1. Introduction
2. Methods
3. Neuroprotective Potential of Cordyceps Extracts
3.1. Cordyceps militaris
3.2. Cordyceps ophioglossoides
3.3. Cordyceps sinensis
3.4. Cordyceps cicadae
| Bioactive Compound | Cordyceps Species | Chemical Class | Biological Activity | References |
|---|---|---|---|---|
| Adenosine | C. sinensis | Nucleoside | Prohibits cancer cell growth Anti-inflammatory effect | [103] |
| Cordycepin | C. sinensis C. militaris C. cicadae | Derivative of the nucleoside adenosine | Enhances immunity Anti-tumor activity Anti-inflammatory Antimicrobial activity | [57,103,121] |
| D-mannitol | C. militaris | Sugar alcohol | Diuretic effects | [57] |
| GABA | C. militaris | Primary amine | Neurotransmitter | [57] |
| Ergotheoneine | C. militaris C. cicadae | Thiourea derivative of histidine | Antioxidant | [57,121] |
| Lovastatin | C. militaris | Statin | Cholesterol-lowering agent | [57] |
| Uridine | C. militaris | Nucleoside | Maintenance of the cellular metabolism | [61] |
| N-(2-Hydroxyethyl) adenosine | C. cicadae | Derivative of the nucleoside adenosine | Anti-inflammatory activity | [121] |
| Cordycepic acid | C. cicadae | Sugar alcohol | Bacteriostatic activity | [103,121] |
| C. sinensis | Antioxidant | |||
| Beauvericin | C. cicadae | Cyclic hexadepsipeptide | Antimicrobial and antitumor activity | [121] |
| Methyl-2-(5-(3-Hydroxybutyl)furan-2-yl)acetate | C. cicadae | Furane methyl ester | Anti-AChE activity | [121] |
| α-furoic acid | C. cicadae | Carboxylic acid | Anti-AChE activity | [121] |
| 2-(5-(3-Oxobutyl)furan-2-yl) acetate | C. cicadae | Furane methyl ester | Anti-AChE activity | [121] |
| Hercynine | C. cicadae | Histadine derivative | Antioxidant | [121] |
| EPSF | C. sinensis | Polysaccharide | Antioxidant, antitumor | [103] |
| APS | C. sinensis | Polysaccharide | Antioxidant | [103] |
| CPS-1 | C. sinensis | Polysaccharide | Antioxidant | [103] |
| CPS-2 | C. sinensis | Polysaccharide | Inhibits cell proliferation | [103] |
| Ergosterol | C. sinensis | Phytosterol | Antimicrobial activity Cytotoxicity | [103] |
| Cordymin | C. sinensis | Peptide | Antidiabetic | [103] |
| Tryptophan | C. sinensis | Amino acid | Sedative effects | [103] |
| Species | Extract | Study | Model | Study Outcome | Mechanism | Refs. |
|---|---|---|---|---|---|---|
| C. militaris | EtOH | in vitro, in vivo | Aβ(1–42)- induced toxicity in mice and C6 glial cells | Improved cognition, decreased NO and lipid peroxidation, downregulated COX-2 and iNOS, downregulated MAPK/JNK/ERK pathway | Antioxidant, Anti-inflammatory | [72,73,74,76] |
| C. militaris | MeOH | in vitro, in vivo | Neuro 2A, scopolamine-induced memory loss in rats | Promoted neurite outgrowth, increased ACh, improved memory | Increase ACh, neurogenesis | [71] |
| C. militaris | AQ | in vivo | Cerebral ischemia-induced short-term memory impairment in gerbils | Protected neuronal death Increased BDNF and TrkB expression | Anti-apoptotic Antioxidant | [83] |
| C. militaris | AQ | in vivo | D-Gal-induced aging mice | Increased SOD, GPx, GSH Decreased MDA Restoration of memory | Antioxidant | [84] |
| C. militaris | EtOH | in vitro, in vivo | PC12 cells and rat | Increased tyrosine hydroxylase | Upregulation of the dopaminergic system | [77] |
| C. militaris | BuOH | in vivo | MCAO-rat, scopolamine-induced memory loss in rats, spinal cord injury | Inhibited MMP-9, downregulated chemokines, delayed neuronal death | Anti-inflammatory | [80,81] |
| C. militaris | AQ | in vivo | Ischemia-induced death and cognitive impairment in rats | Decreased microglial expression Memory improvement | Anti-inflammatory | [82] |
| C. militaris | NP | in vitro | SH-SY5Y | Enhanced the expression of neuronal proteins Increased expression of dopaminergic-specific genes Decreased expression of PS1, PS2, APP Upregulated ADAM10 and SIRT1 Decreased Aβ secretion | Autophagy, neurogenesis, secretion of dopamine | [85] |
| C. ophioglossoides | MeOH | in vitro, in vivo | Aβ (25–35)-induced SK-N-SH and rats | Decreased oxidative stress Restored memory | Antioxidant | [95] |
| C. sinensis | AQ, EtOH | in vitro | Hypoxia-induced oxidative stress in HT22 | Increased SOD, GPx, GSH Decreased MDA, IL-6, TNF-α, NF-kB | Antioxidant Anti-inflammatory | [105] |
| C. sinensis | AQ, EtOH | in vivo | MCAO/R | Decreased IL-1β, TNF-α, MPO, ICAM-1, COX-2 and iNOS Suppressed PMNC infiltration | Anti-inflammatory | [107,108] |
| C. sinensis | AQ | in vitro, in vivo | MCAO/R | Decreased Bax, Cyt c, Caspase-3 | Anti-apoptotic | [110] |
| C. sinensis | - | in vivo | Mice mode of MS-EAE | Decreased Th1 | Immunoregulatory | [115] |
| C. sinensis | Fermented | in vivo | rUCCAO mice model | Reduced white matter lesion | Anti-inflammatory | [116] |
| C. sinensis | Fermented | in vivo | MCAO | Decreased TNF-α, IL-1β, IL-6 Increased SOD and ATP Decreased MDA Memory improvement | Antioxidant Anti-inflammatory | [118] |
| C. cicadae | - | in vivo | ONC rat model | Improved retinal ganglion cell density and P1-N2 amplitude | Antioxidant Anti-apoptotic | [131] |
| C. cicadae | BuOH | in vitro | Glutamate induced toxicity in PC12 cells | Increased GPx, SOD Increased cell viability, decreased LDH | Antioxidant Anti-apoptotic | [132,133] |
| C. cicadae | AQ, MeOH | in vitro | LPS-stimulated RAW 264.7 macrophages | Suppressed TLR-4-mediated NF-kB pathway | Anti-inflammatory | [134] |
| C. cicadae | HA | in vivo | Cisplatin-induced toxicity in mice | Reduced IL-6, TNF-α, and IL-1β; decreased AChE and oxidative stress | Antioxidant Anti-inflammatory | [136] |
| C. cicadae | DOW-cultured | in vivo | D-Gal-induced brain damage and memory impairment in rats | Decreased expression of GFAP, PS1 Decreased COX-2, TNF- α, IL-6, IL-1β | Antioxidant Anti-inflammatory | [137] |
4. Neuroprotective Potential of Bioactive Compounds from Cordyceps
4.1. Cordycepin
4.1.1. Neuroprotection in PD
4.1.2. Neuroprotection in AD
4.1.3. Neuroprotection in Ischemic Stroke
4.1.4. Neuroprotection in Multiple Sclerosis
4.1.5. Neuroprotection in Traumatic Brain Injury
| Disease | Study Model | Mechanism | MOA | Refs. |
|---|---|---|---|---|
| PD | 6-OHDA-induced neurotoxicity in PC12 cells | Decreased caspase-3, increased SOD and ψm | Antioxidant activity | [145] |
| Rotenone-induced toxicity in rat model | Decreased Bcl2 expression, increased ψm, decreased caspase-3 | Anti-apoptotic Antioxidant | [146,147] | |
| MPTP-induced PD in rats and PC12 cells | Suppressed TLR4/NF-κB pathway | Anti-inflammatory | [148] | |
| Glutamate-induced oxidative toxicity in HT22 cells | Downregulated caspase-12 Deceased expression of CHOP, Bax, JNK, PER, p38 Reduced ROS and Ca2+ | Anti-apoptotic, Antioxidant, A1AR activation | [157] | |
| LPS-induced BV2 cells | Neurogenesis Downregulated TNF-α, IL-1β, iNOS, Cox2 | Anti-inflammatory Neurogenesis | [149] | |
| LPS-treated C57BL/6J mice and BV2 cells | Suppressed TLR4/NF-κB-mediated NLRP3 inflammasome activation and GSDMD-related pyroptosis Inhibited pore formation in the plasma membrane Reduced the release of pro-inflammatory mediators | Anti-apoptotic Anti-inflammatory | [151] | |
| Hippocampal brain slice from rats | Reduced excitatory synaptic transmission | Synaptic transmission | [155] | |
| AD | Aβ-induced toxicity in primary hippocampal neurons | Downregulated pTau, anti-AChE, reduced ROS and Ca2+ | Anti-apoptotic Antioxidant Enzyme inhibition A1AR activation | [158] |
| APP/PS1 mice model | Microglia/macrophage polarization through CREB | Neurogenesis | [163] | |
| Ischemic Stroke | OGD model | Increased SOD Decreased MDA Suppressed Glu and Asp Decreased MMP3 | Antioxidant | [139] |
| Ischemic damage in gerbils; β-amyloid and ibotenic acid-induced hippocampal CA1 pyramidal neuronal hyperactivity | Reduced 4-hydroxynonenal, delayed membrane depolarization | Antioxidative A1AR activation | [167,168,169,170] | |
| Acute hypobaric hypoxia-induced BBB disruption and cognitive impairment in rats | Increased tight-junction proteins (claudin5, occluding, zonula occludens-1) Inhibited TLR-4/NF-κB/MMP-9 pathway | Anti-inflammatory Antioxidant | [182] | |
| MS | LPS-induced dendritic cells, MS-EAE mice model | Inhibited AKT/ERK/NF-kB pathway Decreased integrin (β1,α-4), c-type lectin, ICAM1, CCR7 Decreased chemokines Decreased INF-γ, IL-6, IL-17, TNF-α | Antioxidant, Anti-inflammatory | [174] |
| CPZ-induced demyelination in mice | Decreased IL-6, IL-1β Increased IL-4, IL-10, and TGF-β Upregulated BDNF Promoted remyelination | Anti-inflammatory | [173] | |
| TBI | TBI-mice, rats | Decreased MMP-2, MMP-9; CD-16, IL-17, NOX1, MPO, iNOS Increased ZO-1, CD-206, IL-10, IL-1β, Arginase-1 Suppressed neutrophil infiltration | Anti-inflammatory Antioxidant | [178,179] |
4.2. N6-(2-Hydroxyethyl)-Adenosine (HEA)
4.3. Adenosine
4.4. Polysaccharides
4.5. Ergosta-7, 9 (11), 22-Trien-3β-ol (EK100)
4.6. Cordymin
4.7. Active Polypeptide
4.8. Fingolimod
| Name | Nature | Study | Model | Study Outcome | Mechanism | Refs. |
|---|---|---|---|---|---|---|
| N6-(2-hydroxyethyl)-adenosine | Nucleoside | in vitro | H2O2-induced oxidative stress in PC12 cells | Reduced IL-6, IL-1β, TNF-α and NF-kB Reduced LDH release, increased Ψm | Antioxidant Anti-inflammatory | [185] |
| in vitro | LPS-induced inflammation in RAW264.7 macrophages | Decreased pro-inflammatory cytokines by suppressing TLR-4/NF-kB pathway | Anti-inflammatory | [126] | ||
| Adenosine | Nucleoside | in vitro | Glutamate-induced toxicity in PC12 cells | Increased GSH-Px and SOD Increased Bcl-2/Bax ratio Reduced the expression of ERK, p38, and JNK, increased Ψm | Antioxidant Anti-inflammatory Anti-apoptotic | [133] |
| Mixture | Polysaccharide | in vivo | D-Gal-induced aging mice model | Decreased ROS Increased antioxidant enzymes Protected mitochondria | Antioxidant Anti-aging | [84] |
| CPA-1, CPA-2 | Polysaccharide | in vitro | Glutamate-induced toxicity in PC12 cells | Increased cell viability; ncreased GSH-Px, and SOD Reduced LDH release, ROS, and Ca2+ levels | Antioxidant | [193] |
| CP, NP | Polysaccharide | in vitro | LPS-induced inflammation in RAW264.7 macrophages | Inhibited NO, IL-1β, TNF-α | Anti-inflammatory | [196] |
| CP70 | Polysaccharide | in vivo | Drosophila | Increased CAT, SOD expression | Antioxidant Anti-aging | [197] |
| APS | Polysaccharide | in vitro | H2O2-induced stress in PC12 | Increased cell viability; increased GSH-Px, and SOD Reduced LDH release, ROS, and Ca2+ levels | Antioxidant | [194] |
| Ergosta-7, 9 (11), 22-trien-3β-ol | Ergosterol | in vivo | Drosophila AD model | Reduced microglia activation and inflammatory markers | Anti-inflammatory | [204] |
| in vitro | LPS-induced RAW264.7 and BV2 cells | Reduced the cytokine release and pro-inflammatory markers Suppressed TLR4/NF-kB pathway, activated Nrf2/HO-1 pathway | Antioxidant Anti-inflammatory | [67,206] | ||
| in vivo | Ischemic stroke brain injury in mice | Increased neurogenesis, upregulated PI3K/AKT pathway | Anti-inflammatory Anti-apoptotic | [208] | ||
| in vivo, in vitro | Collagenase-induced ICH in mice, BV2 cells | Downregulated MMP-9, COX-2 | Anti-inflammatory | [212] | ||
| Cordymin | Peptide | in vivo | Ischemic stroke brain injury in mice | Elevated GSH Reduced MDA, IL-1β, TNF-α Reduced infiltration of PMNCs | Antioxidant Anti-inflammatory | [213] |
| Active polypeptide | Peptide | in vivo | Scopolamine-induced memory impairment in mice | Increased SOD, Na-K-ATPase Decreased MDA and AChE Increased secretion of neurotransmitters | Antioxidant Anti-inflammatory Anti-apoptotic | [219] |
| Fingolimod | Myriocin synthetic analog | in vivo, in vitro | Focal CI/RI in the rat, mice PD model 6-OHDG Rotenone-induced SH-SY5Y Cells | Protected BBB Improved neurological deficits Reduced IL-17 Reduced caspase-3 expression | Immunosuppressant Anti-inflammatory Anti-apoptotic | [225,226,229] |
5. Safety and Toxicity
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, D.; Ying, J.; Wang, X.; Zhao, T.; Yoon, S.; Fang, Y.; Zheng, Q.; Liu, X.; Yu, W.; Hua, F. Mitochondrial Dynamics: A Key Role in Neurodegeneration and a Potential Target for Neurodegenerative Disease. Front. Neurosci. 2021, 15, 654785. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; O, W.; Li, W.; Jiang, Z.-G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, A.; Jose Egea-Guerrero, J.; Murillo-Cabezas, F.; Carrillo-Vico, A. Oxidative stress in traumatic brain injury. Curr. Med. Chem. 2014, 21, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Keeler, J.L.; Patsalos, O.; Chung, R.; Schmidt, U.; Breen, G.; Treasure, J.; Himmerich, H.; Dalton, B. Short communication: Serum levels of brain-derived neurotrophic factor and association with pro-inflammatory cytokines in acute and recovered anorexia nervosa. J. Psychiatr. Res. 2022, 150, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Hong, J.S. Why neurodegenerative diseases are progressive: Uncontrolled inflammation drives disease progression. Trends Immunol. 2008, 29, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Liu, B.; Zhang, W.; Hong, J.S. Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol. Sci. 2003, 24, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Ziv, Y. Immunity to self and self-maintenance: A unified theory of brain pathologies. Trends Immunol. 2008, 29, 211–219. [Google Scholar] [CrossRef]
- Liu, B.; Hong, J.S. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003, 304, 1–7. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [PubMed]
- Das Sarma, J. Microglia-mediated neuroinflammation is an amplifier of virus-induced neuropathology. J. Neurovirol. 2014, 20, 122–136. [Google Scholar] [CrossRef]
- Lian, L.; Zhang, Y.; Liu, L.; Yang, L.; Cai, Y.; Zhang, J.; Xu, S. Neuroinflammation in Ischemic Stroke: Focus on MicroRNA-mediated Polarization of Microglia. Front. Mol. Neurosci. 2020, 13, 612439. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Srivastava, A.K.; Arnold, W.D.; Singh, M.P.; Zhang, Y. Neurodegeneration: Etiologies and new therapies. Biomed. Res. Int. 2015, 2015, 272630. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Y.; Shen, Y.; Zhang, Y.; Liu, L.; Yang, X. Dietary polyphenols: Regulate the advanced glycation end products-RAGE axis and the microbiota-gut-brain axis to prevent neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2023, 63, 9816–9842. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, Y.; Chen, Q.; Liu, Y.; Qiao, Z.; Sang, S.; Zhang, J.; Zhan, S.; Wu, Z.; Liu, L. Research advances of advanced glycation end products in milk and dairy products: Formation, determination, control strategy and immunometabolism via gut microbiota. Food Chem. 2023, 417, 135861. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Y.; Dong, L.; Shi, R.; Wu, Z.; Liu, L.; Zhang, J.; Wu, Z.; Pan, D. Quantitative determination of Nε-(carboxymethyl) lysine in sterilized milk by isotope dilution UPLC-MS/MS method without derivatization and ion pair reagents. Food Chem. 2022, 385, 132697. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Aryal, P.; Soni, P. RAGE Inhibitors in Neurodegenerative Diseases. Biomedicines 2023, 11, 1131. [Google Scholar] [CrossRef]
- Mushrooms, R. Cordyceps Sinensis vs Militaris: What’s the Best Cordyceps Supplement? 2023. Available online: https://www.realmushrooms.com/cordyceps-sinensis-vs-militaris/ (accessed on 20 August 2023).
- Kobayasi, Y. The genus Cordyceps and its allies. Sci. Rep. Tokyo Bunrika Daigaku Sec. B 1941, 84, 53–260. [Google Scholar]
- Zhang, Y.; Li, E.; Wang, C.; Li, Y.; Liu, X. Ophiocordyceps sinensis, the flagship fungus of China: Terminology, life strategy and ecology. Mycology 2012, 3, 2–10. [Google Scholar]
- Lin, W.-J.; Lee, Y.-I.; Liu, S.-L.; Lin, C.-C.; Chung, T.-Y.; Chou, J.-Y. Evaluating the tradeoffs of a generalist parasitoid fungus, Ophiocordyceps unilateralis, on different sympatric ant hosts. Sci. Rep. 2020, 10, 6428. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.R. Cordyceps: A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry 2008, 69, 1469–1495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Xia, Q.; Liu, L.; Wu, Z.; Pan, D. Recent advances of cereal β-glucan on immunity with gut microbiota regulation functions and its intelligent gelling application. Crit. Rev. Food Sci. Nutr. 2023, 63, 3895–3911. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-S.; Halpern, G.M.; Jones, K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis Part II. J. Altern. Complement. Med. 1998, 4, 429–457. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Park, S.J.; Park, Y.J. The Anticancer Properties of Cordycepin and Their Underlying Mechanisms. Int. J. Mol. Sci. 2018, 19, 3027. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Lee, S.; Lee, K.; Shin, Y.S.; Kang, H.; Cho, H. Anti-cancer effect of Cordyceps militaris in human colorectal carcinoma RKO cells via cell cycle arrest and mitochondrial apoptosis. Daru 2015, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Jang, H.J.; Shen, L.; Yang, K.E.; Jang, M.S.; Huh, Y.H.; Yoo, H.S.; Park, J.; Jang, I.S.; Park, S.J. Cordyceps militaris Exerts Anticancer Effect on Non-Small Cell Lung Cancer by Inhibiting Hedgehog Signaling via Suppression of TCTN3. Integr. Cancer Ther. 2020, 19, 1534735420923756. [Google Scholar] [CrossRef]
- Liu, X.; Dun, M.; Jian, T.; Sun, Y.; Wang, M.; Zhang, G.; Ling, J. Cordyceps militaris extracts and cordycepin ameliorate type 2 diabetes mellitus by modulating the gut microbiota and metabolites. Front. Pharmacol. 2023, 14, 1134429. [Google Scholar] [CrossRef]
- Dong, Y.; Jing, T.; Meng, Q.; Liu, C.; Hu, S.; Ma, Y.; Liu, Y.; Lu, J.; Cheng, Y.; Wang, D.; et al. Studies on the antidiabetic activities of Cordyceps militaris extract in diet-streptozotocin-induced diabetic Sprague-Dawley rats. Biomed. Res. Int. 2014, 2014, 160980. [Google Scholar] [CrossRef]
- Hirsch, K.R.; Smith-Ryan, A.E.; Roelofs, E.J.; Trexler, E.T.; Mock, M.G. Cordyceps militaris Improves Tolerance to High-Intensity Exercise After Acute and Chronic Supplementation. J. Diet. Suppl. 2017, 14, 42–53. [Google Scholar] [CrossRef]
- Nguyen, Q.-V.; Vu, T.-T.; Tran, M.-T.; Ho Thi, P.T.; Thu, H.; Le Thi, T.H.; Chuyen, H.V.; Dinh, M.-H. Antioxidant activity and hepatoprotective effect of exopolysaccharides from cultivated ophiocordyceps sinensis against CCl4-induced liver damages. Nat. Prod. Commun. 2021, 16, 1934578X21997670. [Google Scholar] [CrossRef]
- Chang, Y.; Jeng, K.-C.; Huang, K.-F.; Lee, Y.-C.; Hou, C.-W.; Chen, K.-H.; Cheng, F.-Y.; Liao, J.-W.; Chen, Y.-S. Effect of Cordyceps militaris supplementation on sperm production, sperm motility and hormones in Sprague-Dawley rats. Am. J. Chin. Med. 2008, 36, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Q.; Yin, F.; Shen, N.; Lin, P.; Xia, B.; Li, Y.J.; Guo, S.D. Polysaccharide CM1 from Cordyceps militaris hinders adipocyte differentiation and alleviates hyperlipidemia in LDLR((+/−)) hamsters. Lipids Health Dis. 2021, 20, 178. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Kai, Q.; Gao, J.; Lian, Z.Q.; Wu, C.M.; Wu, C.A.; Zhu, H.B. Cordycepin prevents hyperlipidemia in hamsters fed a high-fat diet via activation of AMP-activated protein kinase. J. Pharmacol. Sci. 2010, 113, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Solana, M.A.; Corral-Guerrero, I.A.; Téllez-Valencia, A.; Avitia-Domínguez, C.; Meza-Velázquez, J.A.; de Casa, A.G.; Sierra-Campos, E. Cordyceps militaris Inhibited Angiotensin-Converting Enzyme through Molecular Interaction between Cordycepin and ACE C-Domain. Life 2022, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, H.; Yang, M.; Xu, Y.; Hou, L.; Yu, H.; Wang, X.; Zhang, Z.; Han, J. Bidirectional regulatory effects of Cordyceps on arrhythmia: Clinical evaluations and network pharmacology. Front. Pharmacol. 2022, 13, 948173. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Liang, H.; Liu, L.; Li, S.; Chen, J.; Xie, Y. Transcriptomic analysis of the anti-inflammatory effect of Cordyceps militaris extract on acute gouty arthritis. Front. Pharmacol. 2022, 13, 1035101. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Baggio, C.H.; Borato, D.G.; Santana-Filho, A.P.; Sassaki, G.L.; Iacomini, M.; Van Griensven, L.J. Anti-inflammatory properties of the medicinal mushroom Cordyceps militaris might be related to its linear (1→3)-β-D-glucan. PLoS ONE 2014, 9, e110266. [Google Scholar] [CrossRef]
- Tan, W.; Wang, Y.; Dai, H.; Deng, J.; Wu, Z.; Lin, L.; Yang, J. Potential Therapeutic Strategies for Renal Fibrosis: Cordyceps and Related Products. Front. Pharmacol. 2022, 13, 932172. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Lu, Y.; Zhang, J.; Yang, M.; Tian, Y.; Dong, J.; Liao, L. Protective effect of Cordyceps sinensis against diabetic kidney disease through promoting proliferation and inhibiting apoptosis of renal proximal tubular cells. BMC Complement. Med. Ther. 2023, 23, 109. [Google Scholar] [CrossRef]
- Sun, T.; Dong, W.; Jiang, G.; Yang, J.; Liu, J.; Zhao, L.; Ma, P. Cordyceps militaris Improves Chronic Kidney Disease by Affecting TLR4/NF-κB Redox Signaling Pathway. Oxid. Med. Cell Longev. 2019, 2019, 7850863. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cheung, F.W.; Chan, M.H.; Hui, P.K.; Ip, S.P.; Ling, Y.H.; Che, C.T.; Liu, W.K. Protective roles of Cordyceps on lung fibrosis in cellular and rat models. J. Ethnopharmacol. 2012, 143, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Huang, K.S.; Shaw, J.F.; Chen, J.R.; Kuo, W.S.; Shen, G.; Grumezescu, A.M.; Holban, A.M.; Wang, Y.T.; Wang, J.S.; et al. Trends in the Immunomodulatory Effects of Cordyceps militaris: Total Extracts, Polysaccharides and Cordycepin. Front. Pharmacol. 2020, 11, 575704. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Shin, H.S.; Leyva-Gómez, G.; Prado-Audelo, M.L.D.; Cortes, H.; Singh, Y.D.; Panda, M.K.; Mishra, A.P.; Nigam, M.; Saklani, S.; et al. Cordyceps spp.: A Review on Its Immune-Stimulatory and Other Biological Potentials. Front. Pharmacol. 2020, 11, 602364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gong, Z.; Su, Y.; Lin, J.; Tang, K. Cordyceps fungi: Natural products, pharmacological functions and developmental products. J. Pharm. Pharmacol. 2009, 61, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xie, J.; Wang, L.; Li, S. Advanced development in chemical analysis of Cordyceps. J. Pharm. Biomed. Anal. 2014, 87, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gao, Y.; Zhou, Y.; Yu, F.; Li, X.; Zhang, N. Mechanism of Cordyceps sinensis and its Extracts in the Treatment of Diabetic Kidney Disease: A Review. Front. Pharmacol. 2022, 13, 881835. [Google Scholar] [CrossRef] [PubMed]
- Shashidhar, M.G.; Giridhar, P.; Udaya Sankar, K.; Manohar, B. Bioactive principles from Cordyceps sinensis: A potent food supplement—A review. J. Funct. Foods 2013, 5, 1013–1030. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sandhu, S.S.; Sharma, A.K. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Shweta; Abdullah, S.; Komal; Kumar, A. A brief review on the medicinal uses of Cordyceps militaris. Pharmacol. Res. Mod. Chin. Med. 2023, 7, 100228. [Google Scholar] [CrossRef]
- Yue, K.; Ye, M.; Zhou, Z.; Sun, W.; Lin, X. The genus Cordyceps: A chemical and pharmacological review. J. Pharm. Pharmacol. 2012, 65, 474–493. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.-R.; Ahmed, M.; Park, H.-J. Cordyceps militaris as a Bio Functional Food Source: Pharmacological Potential, Anti-Inflammatory Actions and Related Molecular Mechanisms. Microorganisms 2022, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Siddiqui, A.J.; Patel, M.; Awadelkareem, A.M.; Snoussi, M.; Ashraf, M.S.; Adnan, M.; Hadi, S. Cordycepin for Health and Wellbeing: A Potent Bioactive Metabolite of an Entomopathogenic Cordyceps Medicinal Fungus and Its Nutraceutical and Therapeutic Potential. Molecules 2020, 25, 2735. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, W.A.; Daba, G. Cordyceps more than edible mushroom—A rich source of diverse bioactive metabolites with huge medicinal benefits. J. Biomed. Res. Environ. Sci. 2022, 3, 566–574. [Google Scholar] [CrossRef]
- Jędrejko, K.J.; Lazur, J.; Muszyńska, B. Cordyceps militaris: An Overview of Its Chemical Constituents in Relation to Biological Activity. Foods 2021, 10, 2634. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.X.; Wang, S.; Nie, S.; Marcone, M. Properties of Cordyceps sinensis: A review. J. Funct. Foods 2013, 5, 550–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, C.; Duan, Y.; Zhang, H.; Ma, H. Advance in Cordyceps militaris (Linn) Link polysaccharides: Isolation, structure, and bioactivities: A review. Int. J. Biol. Macromol. 2019, 132, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.T.; Yang, Y.C.; Li, Y.H.; Mau, J.L.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef]
- Deacon, J.W. Fungal Biology; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Chan, J.S.; Barseghyan, G.S.; Asatiani, M.D.; Wasser, S.P. Chemical Composition and Medicinal Value of Fruiting Bodies and Submerged Cultured Mycelia of Caterpillar Medicinal Fungus Cordyceps militaris CBS-132098 (Ascomycetes). Int. J. Med. Mushrooms 2015, 17, 649–659. [Google Scholar] [CrossRef]
- Wu, X.-F.; Zhang, M.; Li, Z. Influence of infrared drying on the drying kinetics, bioactive compounds and flavor of Cordyceps militaris. LWT 2019, 111, 790–798. [Google Scholar] [CrossRef]
- Xia, Y.; Luo, F.; Shang, Y.; Chen, P.; Lu, Y.; Wang, C. Fungal Cordycepin Biosynthesis Is Coupled with the Production of the Safeguard Molecule Pentostatin. Cell Chem. Biol. 2017, 24, 1479–1489.e4. [Google Scholar] [CrossRef] [PubMed]
- Margolis, J.; Grever, M.R. Pentostatin (Nipent): A review of potential toxicity and its management. Semin Oncol. 2000, 27, 9–14. [Google Scholar] [PubMed]
- Wong, J.H.; Ng, T.B.; Wang, H.; Sze, S.C.; Zhang, K.Y.; Li, Q.; Lu, X. Cordymin, an antifungal peptide from the medicinal fungus Cordyceps militaris. Phytomedicine 2011, 18, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-P.; Chen, D.-R.; Lin, W.-J.; Lin, Y.-H.; Chen, J.-Y.; Kuo, Y.-H.; Chung, J.-G.; Hsia, T.-C.; Hsieh, W.-T. Ergosta-7, 9 (11), 22-trien-3β-ol attenuates inflammatory responses via inhibiting MAPK/AP-1 induced IL-6/JAK/STAT pathways and activating Nrf2/HO-1 signaling in LPS-stimulated macrophage-like cells. Antioxidants 2021, 10, 1430. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V.; Buccafusco, J.J. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef] [PubMed]
- De Jaco, A.; Augusti-Tocco, G.; Biagioni, S. Muscarinic acetylcholine receptors induce neurite outgrowth and activate the synapsin I gene promoter in neuroblastoma clones. Neuroscience 2002, 113, 331–338. [Google Scholar] [CrossRef]
- Anelli, T.; Mannello, F.; Salani, M.; Tonti, G.A.; Poiana, G.; Biagioni, S. Acetylcholine induces neurite outgrowth and modulates matrix metalloproteinase 2 and 9. Biochem. Biophys. Res. Commun. 2007, 362, 269–274. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.; Park, J.; Shin, H.-J.; Kwon, S.; Yeom, M.; Sur, B.; Kim, S.; Kim, M.; Lee, H. Cordyceps militaris improves neurite outgrowth in Neuro2a cells and reverses memory impairment in rats. Food Sci. Biotechnol. 2011, 20, 1599–1608. [Google Scholar] [CrossRef]
- Phan, C.-W.; David, P.; Naidu, M.; Wong, K.-H.; Sabaratnam, V. Neurite outgrowth stimulatory effects of culinary-medicinal mushrooms and their toxicity assessment using differentiating Neuro-2a and embryonic fibroblast BALB/3T3. BMC Complement. Altern. Med. 2013, 13, 261. [Google Scholar] [CrossRef]
- He, M.T.; Park, C.H.; Cho, E.J. Caterpillar Medicinal: Mushroom, Cordyceps militaris (Ascomycota), Attenuates Aβ 1− 42− Induced Amyloidogenesis and Inflammatory Response by Suppressing Amyloid Precursor Protein Progression and p38 MAPK/JNK Activation. Int. J. Med. Mushrooms 2021, 23, 2634. [Google Scholar] [CrossRef]
- He, M.T.; Lee, A.Y.; Park, C.H.; Cho, E.J. Protective effect of Cordyceps militaris against hydrogen peroxide-induced oxidative stress in vitro. Nutr. Res. Pr. 2019, 13, 279–285. [Google Scholar] [CrossRef]
- Bogoyevitch, M.A.; Kobe, B. Uses for JNK: The many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 2006, 70, 1061–1095. [Google Scholar] [CrossRef] [PubMed]
- He, M.T.; Lee, A.Y.; Kim, J.H.; Park, C.H.; Shin, Y.S.; Cho, E.J. Protective role of Cordyceps militaris in Aβ(1-42)-induced Alzheimer’s disease in vivo. Food Sci. Biotechnol. 2019, 28, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, K.; Kim, S.; Park, Y.L.; Choi, B.-S.; Park, S.-E.; Kim, S.-J. Enhancement of tyrosine hydroxylase expression by Cordyceps militaris. Cent. Eur. J. Biol. 2010, 5, 214–223. [Google Scholar] [CrossRef]
- Lucke-Wold, B.P.; Logsdon, A.F.; Turner, R.C.; Rosen, C.L.; Huber, J.D. Chapter Fourteen—Aging, the Metabolic Syndrome, and Ischemic Stroke: Redefining the Approach for Studying the Blood–Brain Barrier in a Complex Neurological Disease. In Advances in Pharmacology; Davis, T.P., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 411–449. [Google Scholar]
- Pluta, R.; Furmaga-Jabłońska, W.; Januszewski, S.; Czuczwar, S.J. Post-ischemic brain neurodegeneration in the form of Alzheimer’s disease proteinopathy: Possible therapeutic role of curcumin. Nutrients 2022, 14, 248. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Cho, G.-S.; Ryu, S.; Kim, H.J.; Song, H.Y.; Yune, T.Y.; Ju, C.; Kim, W.-K. Post-ischemic treatment of WIB801C, standardized Cordyceps extract, reduces cerebral ischemic injury via inhibition of inflammatory cell migration. J. Ethnopharmacol. 2016, 186, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choi, H.Y.; Baik, H.H.; Ju, B.G.; Kim, W.K.; Yune, T.Y. Cordycepin-enriched WIB-801C from Cordyceps militaris improves functional recovery by attenuating blood-spinal cord barrier disruption after spinal cord injury. J. Ethnopharmacol. 2017, 203, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Kim, H.J.; Abu-Taweel, G.M.; Oh, J.; Sung, G.H. Neuroprotective and therapeutic effect of Cordyceps militaris on ischemia-induced neuronal death and cognitive impairments. Saudi J. Biol. Sci. 2019, 26, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Ko, I.-G.; Kim, S.-E.; Hwang, L.; Jin, J.-J.; Choi, H.-H.; Kim, C.-J. Aqueous extract of Cordyceps alleviates cerebral ischemia-induced short-term memory impairment in gerbils. J. Exerc. Rehabil. 2016, 12, 69. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Zhang, J.; Jia, J.; Ding, J.; Luo, R.; Liu, Z. Cordyceps militaris extract attenuates D-galactose-induced memory impairment in mice. J. Med. Food 2012, 15, 1057–1063. [Google Scholar] [CrossRef]
- Kaokaen, P.; Sorraksa, N.; Phonchai, R.; Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Enhancing Neurological Competence of Nanoencapsulated Cordyceps/Turmeric Extracts in Human Neuroblastoma SH-SY5Y Cells. Cell Mol. Bioeng. 2023, 16, 81–93. [Google Scholar] [CrossRef]
- Kikkawa, T.; Casingal, C.R.; Chun, S.H.; Shinohara, H.; Hiraoka, K.; Osumi, N. The role of Pax6 in brain development and its impact on pathogenesis of autism spectrum disorder. Brain Res. 2019, 1705, 95–103. [Google Scholar] [CrossRef]
- Krishnasamy, S.; Weng, Y.-C.; Thammisetty, S.S.; Phaneuf, D.; Lalancette-Hebert, M.; Kriz, J. Molecular imaging of nestin in neuroinflammatory conditions reveals marked signal induction in activated microglia. J. Neuroinflamm. 2017, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rahman, M.H.; Mamun-Or-Rashid, A.; Hwang, H.; Chung, S.; Kim, B.; Rhim, H. Autophagy modulation in aggresome formation: Emerging implications and treatments of Alzheimer’s disease. Biomedicines 2022, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
- Kneifel, H.; Konig, W.A.; Loeffler, W.; Müller, R. Ophiocordin, an antifungal antibiotic of Cordyceps ophioglossoides. Arch. Microbiol. 1977, 113, 121–130. [Google Scholar] [CrossRef]
- Braeuer, S.; Borovička, J.; Glabonjat, R.A.; Steiner, L.; Goessler, W. Arsenocholine-O-sulfate: A novel compound as major arsenic species in the parasitic mushroom Tolypocladium ophioglossoides. Chemosphere 2021, 265, 128886. [Google Scholar] [CrossRef] [PubMed]
- Quandt, C.A.; Bushley, K.E.; Spatafora, J.W. The genome of the truffle-parasite Tolypocladium ophioglossoides and the evolution of antifungal peptaibiotics. BMC Genom. 2015, 16, 553. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, M.; Guo, Y.-Y.; Mao, X.-M.; Chen, X.-A.; Li, Y.-Q. Revelation of the balanol biosynthetic pathway in Tolypocladium ophioglossoides. Org. Lett. 2018, 20, 6323–6326. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Z.; Sun, Y.; Ding, Z.; LÜ, L.; Li, Y. Optimization for Production of Intracellular Polysaccharide from Cordyceps ophioglossoides L2 in Submerged Culture and Its Antioxidant Activities in vitro. Chin. J. Chem. Eng. 2012, 20, 294–301. [Google Scholar] [CrossRef]
- Sato, K.; Wakamiya, A.; Maeda, T.; Noguchi, K.; Takashima, A.; Imahori, K. Correlation among secondary structure, amyloid precursor protein accumulation, and neurotoxicity of amyloid β (25–35) peptide as analyzed by single alanine substitution. J. Biochem. 1995, 118, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.-Q.; Park, B.-C.; Lee, J.-S.; Choi, H.-D.; Lee, Y.-S.; Yang, J.-H.; Kim, J.-A. Mycelial extract of Cordyceps ophioglossoides prevents neuronal cell death and ameliorates β-amyloid peptide-induced memory deficits in rats. Biol. Pharm. Bull. 2004, 27, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, F.; Tsim, K.W. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharm. Biomed. Anal. 2006, 41, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Zhao, S.Y.; Wang, J.H.; Kuang, H.C.; Liu, X. Distribution of nucleosides and nucleobases in edible fungi. J. Agric. Food Chem. 2008, 56, 809–815. [Google Scholar] [CrossRef]
- Guan, J.; Yang, F.Q.; Li, S.P. Evaluation of carbohydrates in natural and cultured Cordyceps by pressurized liquid extraction and gas chromatography coupled with mass spectrometry. Molecules 2010, 15, 4227–4241. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Li, X.L. Determination of ergosterol in Cordyceps sinensis and Cordyceps black-bone chicken capsules by HPLC. Yao Xue Xue Bao 1991, 26, 768–771. [Google Scholar]
- Li, S.P.; Li, P.; Lai, C.M.; Gong, Y.X.; Kan, K.K.; Dong, T.T.; Tsim, K.W.; Wang, Y.T. Simultaneous determination of ergosterol, nucleosides and their bases from natural and cultured Cordyceps by pressurised liquid extraction and high-performance liquid chromatography. J. Chromatogr. A 2004, 1036, 239–243. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Wang, J.-H.; Liu, X.; Kuang, H.-C.; Zhao, S.-Y. Simultaneous determination of free ergosterol and ergosteryl esters in Cordyceps sinensis by HPLC. Food Chem. 2007, 105, 1755–1759. [Google Scholar] [CrossRef]
- Bok, J.W.; Lermer, L.; Chilton, J.; Klingeman, H.G.; Towers, G.H. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry 1999, 51, 891–898. [Google Scholar] [CrossRef]
- Mishra, R.; Upadhyay, Y. Cordicepssinensis: The Chinese Rasayan-current research scenario. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 1503–1519. [Google Scholar]
- Liu, Y.; Wang, J.; Wang, W.; Zhang, H.; Zhang, X.; Han, C. The Chemical Constituents and Pharmacological Actions of Cordyceps sinensis. Evid.-Based Complement. Altern. Med. 2015, 2015, 575063. [Google Scholar]
- Pal, M.; Bhardwaj, A.; Manickam, M.; Tulsawani, R.; Srivastava, M.; Sugadev, R.; Misra, K. Protective efficacy of the caterpillar mushroom, Ophiocordyceps sinensis (Ascomycetes), from India in neuronal hippocampal cells against hypoxia. Int. J. Med. Mushrooms 2015, 17, 829–840. [Google Scholar] [CrossRef]
- Mehrotra, S.; Kirar, V.; Vats, P.; Nandi, S.P.; Negi, P.; Misra, K. Phytochemical and Antimicrobial Activities of Himalayan Cordyceps Sinensis (Berk.) Sacc; NISCAIR-CSIR: New Delhi, India, 2015. [Google Scholar]
- Liu, Z.; Li, P.; Zhao, D.; Tang, H.; Guo, J. Anti-inflammation Effects of Cordyceps sinensis Mycelium in Focal Cerebral Ischemic Injury Rats. Inflammation 2011, 34, 639–644. [Google Scholar] [CrossRef]
- Kong, R.; Zhang, Y.; Zhang, S.; Liu, M.; Sun, W.; Xing, Y.; Guan, Y.; Han, C.; Liu, Z. Protective Effect of Ethanol Extracts of the Chinese Caterpillar Mushroom, Ophiocordyceps sinensis (Ascomycetes), on the Experimental Middle Cerebral Artery Occlusion/Reperfusion (MCAO/R) Model. Int. J. Med. Mushrooms 2015, 17, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, T.; Wang, T.; Song, J.; Zhou, Z. Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on cerebral ischemia rats. Biomed. Rep. 2013, 1, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Tan, T.-Y.; Li, Y.-X.; Li, Y.; Chen, Y.-F.; Ma, R.; Wang, S.-Y.; Li, Q.; Liu, Z.-Q. The protective effect of cordyceps sinensis extract on cerebral ischemic injury via modulating the mitochondrial respiratory chain and inhibiting the mitochondrial apoptotic pathway. Biomed. Pharmacother. 2020, 124, 109834. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Kamimura, D.; Sabharwal, L.; Yamada, M.; Bando, H.; Ogura, H.; Atsumi, T.; Murakami, M. Regulation of Immune Cell Infiltration into the CNS by Regional Neural Inputs Explained by the Gate Theory. Mediat. Inflamm. 2013, 2013, 898165. [Google Scholar] [CrossRef] [PubMed]
- Goverman, J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009, 9, 393–407. [Google Scholar] [CrossRef]
- Prajeeth, C.K.; Kronisch, J.; Khorooshi, R.; Knier, B.; Toft-Hansen, H.; Gudi, V.; Floess, S.; Huehn, J.; Owens, T.; Korn, T.; et al. Effectors of Th1 and Th17 cells act on astrocytes and augment their neuroinflammatory properties. J. Neuroinflamm. 2017, 14, 204. [Google Scholar] [CrossRef]
- Zhong, S.S.; Xiang, Y.J.; Liu, P.J.; He, Y.; Yang, T.T.; Wang, Y.Y.; Rong, A.; Zhang, J.; Liu, G.Z. Effect of Cordyceps sinensis on the Treatment of Experimental Autoimmune Encephalomyelitis: A Pilot Study on Mice Model. Chin. Med. J. 2017, 130, 2296–2301. [Google Scholar] [PubMed]
- Chen, Y.; Fu, L.; Han, M.; Jin, M.; Wu, J.; Tan, L.; Chen, Z.; Zhang, X. The Prophylactic and Therapeutic Effects of Fermented Cordyceps sinensis Powder, Cs-C-Q80, on Subcortical Ischemic Vascular Dementia in Mice. Evid.-Based Complement. Altern. Med. 2018, 2018, 4362715. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, K.; Adachi, K.; Kataoka, S.; Watanabe, A.; Tabira, T.; Takahashi, K.; Wakita, H. Chronic cerebral hypoperfusion induced by right unilateral common carotid artery occlusion causes delayed white matter lesions and cognitive impairment in adult mice. Exp. Neurol. 2008, 210, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, W.; Wu, X.; Hong, D.; Lu, X.; Rao, Y. Protective effects of Corbrin Capsule against permanent cerebral ischemia in mice. Biomed. Pharmacother. 2020, 121, 109646. [Google Scholar] [CrossRef] [PubMed]
- Li, I.C.; Lin, S.; Tsai, Y.T.; Hsu, J.H.; Chen, Y.L.; Lin, W.H.; Chen, C.C. Cordyceps cicadae mycelia and its active compound HEA exert beneficial effects on blood glucose in type 2 diabetic db/db mice. J. Sci. Food Agric. 2019, 99, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, T.; Li, C.; Xie, L.; Li, N.; Hou, T.; Wang, Y.; Wang, B. Potential therapeutic effects of Cordyceps cicadae and Paecilomyces cicadae on adenine-induced chronic renal failure in rats and their phytochemical analysis. Drug Des. Devel Ther. 2019, 13, 103–117. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Q.; Weng, Q. Secondary metabolites (SMs) of Isaria cicadae and Isaria tenuipes. RSC Adv. 2019, 9, 172–184. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, F.; Cen, K.; Xiao, G.; Yin, Y.; Li, C.; Li, Z.; Zhan, S.; Zhang, H.; Wang, C. Omics data reveal the unusual asexual-fruiting nature and secondary metabolic potentials of the medicinal fungus Cordyceps cicadae. BMC Genom. 2017, 18, 668. [Google Scholar] [CrossRef]
- Chyau, C.-C.; Wu, H.-L.; Peng, C.-C.; Huang, S.-H.; Chen, C.-C.; Chen, C.-H.; Peng, R.Y. Potential protection effect of ER homeostasis of N6-(2-hydroxyethyl) adenosine isolated from Cordyceps cicadae in nonsteroidal anti-inflammatory drug-stimulated human proximal tubular cells. Int. J. Mol. Sci. 2021, 22, 1577. [Google Scholar] [CrossRef]
- Latini, S.; Pedata, F. Adenosine in the central nervous system: Release mechanisms and extracellular concentrations. J. Neurochem. 2001, 79, 463–484. [Google Scholar] [CrossRef]
- Furuya, T.; Hirotani, M.; Matsuzawa, M. N6-(2-hydroxyethyl) adenosine, a biologically active compound from cultured mycelia of Cordyceps and Isaria species. Phytochemistry 1983, 22, 2509–2512. [Google Scholar] [CrossRef]
- Wang, X.; Qin, A.; Xiao, F.; Olatunji, O.J.; Zhang, S.; Pan, D.; Han, W.; Wang, D.; Ni, Y. N(6) -(2-hydroxyethyl)-adenosine from Cordyceps cicadae protects against diabetic kidney disease via alleviation of oxidative stress and inflammation. J. Food Biochem. 2019, 43, e12727. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Kang, J.; Wen, T.; Lei, B.; Hyde, K.D. Cordycepin and N6-(2-hydroxyethyl)-adenosine from Cordyceps pruinosa and their interaction with human serum albumin. PLoS ONE 2015, 10, e0121669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zheng, R.; Deng, Y.; Chen, Y.; Zhang, S. Ergosterol peroxide from Cordyceps cicadae ameliorates TGF-beta1-induced activation of kidney fibroblasts. Phytomedicine 2014, 21, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Yoshida, M.; Ohnishi-Kameyama, M.; Shinmoto, H. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264.7 macrophages and growth of HT29 colon adenocarcinoma cells. Br. J. Pharmacol. 2007, 150, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.P.; McDowell, C.M.; Liu, Y.; Wagner, A.H.; Thole, D.; Faga, B.P.; Wordinger, R.J.; Braun, T.A.; Clark, A.F. Optic nerve crush induces spatial and temporal gene expression patterns in retina and optic nerve of BALB/cJ mice. Mol. Neurodegener. 2014, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.T.; Jhou, B.Y.; Hsu, J.H.; Fu, H.I.; Chen, Y.L.; Shih, Y.C.; Chen, C.C.; Tsai, R.K. Neuroprotective Effects of Cordyceps cicadae (Ascomycetes) Mycelium Extract in the Rat Model of Optic Nerve Crush. Int. J. Med. Mushrooms 2022, 24, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, J.; Wang, D.; Yu, X.; Olatunji, O.J.; Ouyang, Z.; Wei, Y. Neuroprotective effects of butanol fraction of Cordyceps cicadae on glutamate-induced damage in pc12 cells involving oxidative toxicity. Chem. Biodivers. 2018, 15, e1700385. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Ouyang, Z.; Su, Z.; Wang, D.; Yu, X. Neuroprotective effects of adenosine isolated from Cordyceps cicadae against oxidative and ER stress damages induced by glutamate in PC12 cells. Environ. Toxicol. Pharmacol. 2016, 44, 53–61. [Google Scholar] [CrossRef]
- Lu, M.Y.; Chen, C.C.; Lee, L.Y.; Lin, T.W.; Kuo, C.F. N(6)-(2-Hydroxyethyl)adenosine in the Medicinal Mushroom Cordyceps cicadae Attenuates Lipopolysaccharide-Stimulated Pro-inflammatory Responses by Suppressing TLR4-Mediated NF-κB Signaling Pathways. J. Nat. Prod. 2015, 78, 2452–2460. [Google Scholar] [CrossRef]
- Santos, N.A.G.d.; Ferreira, R.S.; Santos, A.C.d. Overview of cisplatin-induced neurotoxicity and ototoxicity, and the protective agents. Food Chem. Toxicol. 2020, 136, 111079. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, S.; Olatunji, O.J.; Wu, Y. Nucleosides rich extract from Cordyceps cicadae alleviated cisplatin-induced neurotoxicity in rats: A behavioral, biochemical and histopathological study. Arab. J. Chem. 2022, 15, 103476. [Google Scholar] [CrossRef]
- Chang, C.Y.; Yang, P.X.; Yu, T.L.; Lee, C.L. Cordyceps cicadae NTTU 868 Mycelia Fermented with Deep Ocean Water Minerals Prevents D-Galactose-Induced Memory Deficits by Inhibiting Oxidative Inflammatory Factors and Aging-Related Risk Factors. Nutrients 2023, 15, 1968. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-S.; Halpern, G.M.; Jones, K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis Part I. J. Altern. Complement. Med. 1998, 4, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; He, W.; Zhou, X.; Lv, Q.; Xu, X.; Yang, S.; Zhao, C.; Guo, L. Cordycepin protects against cerebral ischemia/reperfusion injury in vivo and in vitro. Eur. J. Pharmacol. 2011, 664, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ge, Y.; Sun, L.; Xu, X.; Xie, P.; Zhan, M.; Wang, M.; Dong, Z.; Li, J.; Duan, S. Cordycepin inhibits albumin-induced epithelial-mesenchymal transition of renal tubular epithelial cells by reducing reactive oxygen species production. Free Radic. Res. 2012, 46, 174–183. [Google Scholar] [CrossRef]
- He, Y.T.; Zhang, X.L.; Xie, Y.M.; Xu, Y.X.; Li, J.R. Extraction and antioxidant property in vitro of cordycepin in artificially cultivated Cordyceps militaris. Adv. Mater. Res. 2013, 750, 1593–1596. [Google Scholar] [CrossRef]
- Ramesh, T.; Yoo, S.-K.; Kim, S.-W.; Hwang, S.-Y.; Sohn, S.-H.; Kim, I.-W.; Kim, S.-K. Cordycepin (3′-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp. Gerontol. 2012, 47, 979–987. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, Z.; Meng, Q.; Lu, J.; Wang, N.; Liu, H.; Liang, Q.; Quan, Y.; Wang, D.; Xie, J. Cordycepin affects multiple apoptotic pathways to mediate hepatocellular carcinoma cell death. Anti-Cancer Agents Med. Chem. 2017, 17, 143–149. [Google Scholar] [CrossRef]
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis; Exon Publications: Brisbane City, Australia, 2018; pp. 3–26. [Google Scholar]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Ouyang, Z.; Su, Z. Cordycepin protects PC12 cells against 6-hydroxydopamine induced neurotoxicity via its antioxidant properties. Biomed. Pharmacother. 2016, 81, 7–14. [Google Scholar] [CrossRef]
- Jiang, X.; Tang, P.-C.; Chen, Q.; Zhang, X.; Fan, Y.-Y.; Yu, B.-C.; Gu, X.-X.; Sun, Y.; Ge, X.-Q.; Zhang, X.-L. Cordycepin exerts neuroprotective effects via an anti-apoptotic mechanism based on the mitochondrial pathway in a rotenone-induced parkinsonism rat model. CNS Neurol. Disord. Drug Targets 2019, 18, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Huang, W.M.; Tang, P.C.; Sun, Y.; Zhang, X.; Qiu, L.; Yu, B.C.; Zhang, X.Y.; Hong, Y.X.; He, Y.; et al. Anti-inflammatory and neuroprotective effects of natural cordycepin in rotenone-induced PD models through inhibiting Drp1-mediated mitochondrial fission. Neurotoxicology 2021, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhu, X. Cordycepin mitigates MPTP-induced Parkinson’s disease through inhibiting TLR/NF-κB signaling pathway. Life Sci 2019, 223, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, P.; Ge, H.; Qu, X.; Jin, X. Effects of cordycepin on the microglia-overactivation-induced impairments of growth and development of hippocampal cultured neurons. PLoS ONE 2015, 10, e0125902. [Google Scholar] [CrossRef]
- Berg, A. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of IκB: A mechanism for NK-κB acitivation. Mol. Cell Biol. 1993, 13, 3301. [Google Scholar]
- Sun, Y.; Huang, W.-m.; Tang, P.-c.; Zhang, X.; Zhang, X.-y.; Yu, B.-c.; Fan, Y.-Y.; Ge, X.-q.; Zhang, X.-L. Neuroprotective effects of natural cordycepin on LPS-induced Parkinson’s disease through suppressing TLR4/NF-κB/NLRP3-mediated pyroptosis. J. Funct. Foods 2020, 75, 104274. [Google Scholar] [CrossRef]
- Zou, R.-X.; Gu, X.; Ding, J.-J.; Wang, T.; Bi, N.; Niu, K.; Ge, M.; Chen, X.-T.; Wang, H.-L. Pb exposure induces an imbalance of excitatory and inhibitory synaptic transmission in cultured rat hippocampal neurons. Toxicol. Vitr. 2020, 63, 104742. [Google Scholar] [CrossRef] [PubMed]
- Iovino, L.; Tremblay, M.E.; Civiero, L. Glutamate-induced excitotoxicity in Parkinson’s disease: The role of glial cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef]
- Ahmed, I.; Bose, S.K.; Pavese, N.; Ramlackhansingh, A.; Turkheimer, F.; Hotton, G.; Hammers, A.; Brooks, D.J. Glutamate NMDA receptor dysregulation in Parkinson’s disease with dyskinesias. Brain 2011, 134, 979–986. [Google Scholar] [CrossRef]
- Yao, L.H.; Huang, J.N.; Li, C.H.; Li, H.H.; Yan, W.W.; Cai, Z.L.; Liu, W.X.; Xiao, P. Cordycepin suppresses excitatory synaptic transmission in rat hippocampal slices via a presynaptic mechanism. CNS Neurosci. Ther. 2013, 19, 216–221. [Google Scholar] [CrossRef]
- Wang, J.; Gong, Y.; Tan, H.; Li, W.; Yan, B.; Cheng, C.; Wan, J.; Sun, W.; Yuan, C.; Yao, L.-H. Cordycepin suppresses glutamatergic and GABAergic synaptic transmission through activation of A1 adenosine receptor in rat hippocampal CA1 pyramidal neurons. Biomed. Pharmacother. 2022, 145, 112446. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.L.; Park, S.Y.; Kim, Y.H.; Oh, J.-I.; Lee, S.J.; Park, G. The neuroprotective effects of cordycepin inhibit glutamate-induced oxidative and ER stress-associated apoptosis in hippocampal HT22 cells. Neurotoxicology 2014, 41, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Huang, L.-P.; Li, Y.; Liu, C.; Wang, S.; Meng, W.; Wei, S.; Liu, X.-P.; Gong, Y.; Yao, L.-H. Neuroprotective effects of cordycepin inhibit Aβ-induced apoptosis in hippocampal neurons. Neurotoxicology 2018, 68, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Arbel-Ornath, M.; Hudry, E.; Boivin, J.R.; Hashimoto, T.; Takeda, S.; Kuchibhotla, K.V.; Hou, S.; Lattarulo, C.R.; Belcher, A.M.; Shakerdge, N. Soluble oligomeric amyloid-β induces calcium dyshomeostasis that precedes synapse loss in the living mouse brain. Mol. Neurodegener. 2017, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Sayer, R.; Law, E.; Connelly, P.J.; Breen, K.C. Association of a salivary acetylcholinesterase with Alzheimer’s disease and response to cholinesterase inhibitors. Clin. Biochem. 2004, 37, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Pósfai, B.; Cserép, C.; Orsolits, B.; Dénes, Á. New insights into microglia–neuron interactions: A neuron’s perspective. Neuroscience 2019, 405, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249. [Google Scholar] [CrossRef]
- Jiao, L.; Yu, Z.; Zhong, X.; Yao, W.; Xing, L.; Ma, G.; Shen, J.; Wu, Y.; Du, K.; Liu, J.; et al. Cordycepin improved neuronal synaptic plasticity through CREB-induced NGF upregulation driven by MG-M2 polarization: A microglia-neuron symphony in AD. Biomed. Pharmacother. 2023, 157, 114054. [Google Scholar] [CrossRef]
- L, L.; X, W.; Z, Y. Ischemia-reperfusion Injury in the Brain: Mechanisms and Potential Therapeutic Strategies. Biochem. Pharmacol. 2016, 5, 213. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef]
- Chen, L.S.; Stellrecht, C.M.; Gandhi, V. RNA-directed agent, cordycepin, induces cell death in multiple myeloma cells. Br. J. Haematol. 2008, 140, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.K.; Lim, S.S.; Yoo, K.-Y.; Lee, Y.S.; Kim, H.G.; Kang, I.-J.; Kwon, H.J.; Park, J.; Choi, S.Y.; Won, M.-H. A phytochemically characterized extract of Cordyceps militaris and cordycepin protect hippocampal neurons from ischemic injury in gerbils. Planta Medica 2008, 74, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, X.-P.; Jiang, W.; Zeng, B.; Meng, W.; Huang, L.-P.; Li, Y.-P.; Sun, W.; Yuan, C.-H.; Yao, L.-H. Anti-effects of cordycepin to hypoxia-induced membrane depolarization on hippocampal CA1 pyramidal neuron. Eur. J. Pharmacol. 2017, 796, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.-H.; Wang, J.; Liu, C.; Wei, S.; Li, G.; Wang, S.; Meng, W.; Liu, Z.-B.; Huang, L.-P. Cordycepin protects against β-amyloid and ibotenic acid-induced hippocampal CA1 pyramidal neuronal hyperactivity. Korean J. Physiol. Pharmacol. 2019, 23, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-L.; Wang, C.-Y.; Jiang, Z.-J.; Li, H.-H.; Liu, W.-X.; Gong, L.-W.; Xiao, P.; Li, C.-H. Effects of cordycepin on Y-maze learning task in mice. Eur. J. Pharmacol. 2013, 714, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.S.; Cao, Z.P.; Shang, Y.J.; Liu, Q.Y.; Wu, B.Y.; Liu, W.X.; Li, C.H. Neuroprotection of cordycepin in NMDA-induced excitotoxicity by modulating adenosine A(1) receptors. Eur. J. Pharmacol. 2019, 853, 325–335. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, H.; Bao, H.; Zhang, D.; Feng, L.; Xiao, Y.; Zhu, K.; Hou, Y.; Luo, S.; Zhang, Y. Cordycepin (3′-deoxyadenosine) promotes remyelination via suppression of neuroinflammation in a cuprizone-induced mouse model of demyelination. Int. Immunopharmacol. 2019, 75, 105777. [Google Scholar] [CrossRef]
- Song, Y.-C.; Liu, C.-T.; Lee, H.-J.; Yen, H.-R. Cordycepin prevents and ameliorates experimental autoimmune encephalomyelitis by inhibiting leukocyte infiltration and reducing neuroinflammation. Biochem. Pharmacol. 2022, 197, 114918. [Google Scholar] [CrossRef]
- Paschon, V.; Takada, S.H.; Ikebara, J.M.; Sousa, E.; Raeisossadati, R.; Ulrich, H.; Kihara, A.H. Interplay between exosomes, microRNAs and toll-like receptors in brain disorders. Mol. Neurobiol. 2016, 53, 2016–2028. [Google Scholar] [CrossRef]
- Lee, J.; Hamanaka, G.; Lo, E.H.; Arai, K. Heterogeneity of microglia and their differential roles in white matter pathology. CNS Neurosci. Ther. 2019, 25, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, Y.; Katayama, Y.; Mori, T.; Maeda, T.; Kawamata, T. Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir. Suppl. 2006, 96, 130–133. [Google Scholar] [PubMed]
- Wei, P.; Wang, K.; Luo, C.; Huang, Y.; Misilimu, D.; Wen, H.; Jin, P.; Li, C.; Gong, Y.; Gao, Y. Cordycepin confers long-term neuroprotection via inhibiting neutrophil infiltration and neuroinflammation after traumatic brain injury. J. Neuroinflamm. 2021, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wang, A.; He, Y.; Si, Z.; Xu, S.; Zhang, S.; Wang, K.; Wang, D.; Liu, Y. Cordycepin attenuates traumatic brain injury-induced impairments of blood-brain barrier integrity in rats. Brain Res. Bull. 2016, 127, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Brugniaux, J.V.; Filipponi, T.; Marley, C.J.; Stacey, B.; Soria, R.; Rimoldi, S.F.; Cerny, D.; Rexhaj, E.; Pratali, L. Exaggerated systemic oxidative-inflammatory-nitrosative stress in chronic mountain sickness is associated with cognitive decline and depression. J. Physiol. 2019, 597, 611–629. [Google Scholar] [CrossRef]
- Goodman, M.D.; Makley, A.T.; Huber, N.L.; Clarke, C.N.; Friend, L.A.W.; Schuster, R.M.; Bailey, S.R.; Barnes, S.L.; Dorlac, W.C.; Johannigman, J.A. Hypobaric hypoxia exacerbates the neuroinflammatory response to traumatic brain injury. J. Surg. Res. 2011, 165, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Pan, L.; Cui, L.; Li, T.; Zhao, S.; Hu, Y.; Tao, X.; Deng, H.; Jiang, J.; Zhao, B.; et al. Cordycepin ameliorates acute hypobaric hypoxia induced blood-brain barrier disruption, and cognitive impairment partly by suppressing the TLR4/NF-κB/MMP-9 pathway in the adult rats. Eur. J. Pharmacol. 2022, 924, 174952. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, X.; Yang, W.; Yu, L.; Jiang, X.; Chen, Y.; Shen, Z.; Li, C.; Gu, M.; Shi, L. N6-(2-hydroxyethyl)-Adenosine Induces Apoptosis via ER Stress and Autophagy of Gastric Carcinoma Cells In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 5815. [Google Scholar] [CrossRef]
- Lu, T.-H.; Chang, J.-W.; Jhou, B.-Y.; Hsu, J.-H.; Li, T.-J.; Lee, L.-Y.; Chen, Y.-L.; Chang, H.-H.; Chen, C.-C.; Wu, P.-S.; et al. Preventative Effects of Cordyceps cicadae Mycelial Extracts on the Early-Stage Development of Cataracts in UVB-Induced Mice Cataract Model. Nutrients 2023, 15, 3103. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, T.; Olatunji, O.J.; Tang, J.; Wei, Y.; Ouyang, Z. N(6)-(2-hydroxyethyl)-adenosine from Cordyceps cicadae attenuates hydrogen peroxide induced oxidative toxicity in PC12 cells. Metab. Brain Dis. 2019, 34, 1325–1334. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, H.; Qiu, X.; Xu, D.; Chen, Y.; Barnes, G.N.; Tu, Y.; Gyabaah, A.T.; Gharbal, A.H.A.A.; Peng, C. Neuroprotective effects of adenosine A1 receptor signaling on cognitive impairment induced by chronic intermittent hypoxia in mice. Front. Cell Neurosci. 2020, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.K.; Li, J.; Cheung, A.W.; Zhu, Y.; Zheng, K.Y.; Bi, C.W.; Duan, R.; Choi, R.C.; Lau, D.T.; Dong, T.T. Cordysinocan, a polysaccharide isolated from cultured Cordyceps, activates immune responses in cultured T-lymphocytes and macrophages: Signaling cascade and induction of cytokines. J. Ethnopharmacol. 2009, 124, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yan, H.; Ma, X.; Jia, J.; Zhang, G.; Guo, X.; Gui, Z. Comparison of the structural characterization and biological activity of acidic polysaccharides from Cordyceps militaris cultured with different media. World J. Microbiol. Biotechnol. 2012, 28, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Song, L.; Zhao, Y.; Bin, W.; Wang, L.; Zhang, H.; Wu, Y.; Ye, W.; Yao, X. Isolation and biological properties of polysaccharide CPS-1 from cultured Cordyceps militaris. Fitoterapia 2004, 75, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, S.W.; Cheung, P.C.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Liu, J.-y.; Feng, C.-p.; Li, X.; Chang, M.-c.; Meng, J.-l.; Xu, L.-j. Immunomodulatory and antioxidative activity of Cordyceps militaris polysaccharides in mice. Int. J. Biol. Macromol. 2016, 86, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-T.; Li, H.-C.; Li, C.-B.; Dou, D.-Q.; Gao, M.-B. Protective effects on mitochondria and anti-aging activity of polysaccharides from cultivated fruiting bodies of Cordyceps militaris. Am. J. Chin. Med. 2010, 38, 1093–1106. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Wei, Y.; Ouyang, Z.; Su, Z. Polysaccharides purified from Cordyceps cicadae protects PC12 cells against glutamate-induced oxidative damage. Carbohydr. Polym. 2016, 153, 187–195. [Google Scholar] [CrossRef]
- Shen, W.; Song, D.; Wu, J.; Zhang, W. Protective effect of a polysaccharide isolated from a cultivated Cordyceps mycelia on hydrogen peroxide-induced oxidative damage in PC12 cells. Phytother. Res. 2011, 25, 675–680. [Google Scholar] [CrossRef]
- Li, S.P.; Zhao, K.J.; Ji, Z.N.; Song, Z.H.; Dong, T.T.X.; Lo, C.K.; Cheung, J.K.H.; Zhu, S.Q.; Tsim, K.W.K. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003, 73, 2503–2513. [Google Scholar] [CrossRef]
- Yang, C.H.; Su, C.H.; Liu, S.C.; Ng, L.T. Isolation, Anti-Inflammatory Activity and Physico-chemical Properties of Bioactive Polysaccharides from Fruiting Bodies of Cultivated Cordyceps cicadae (Ascomycetes). Int. J. Med. Mushrooms 2019, 21, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, X.; Ge, Q.; Li, J.; Wang, D.; Wei, Y.; Ouyang, Z. Antioxidant and anti-aging activities of polysaccharides from Cordyceps cicadae. Int. J. Biol. Macromol. 2020, 157, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, H.; Nakamura, E.; Okuyama, E.; Ishibashi, M. Six immunosuppressive features from an ascomycete, Zopfiella longicaudata, found in a screening study monitored by immunomodulatory activity. Chem. Pharm. Bull. 2004, 52, 1005–1008. [Google Scholar] [CrossRef]
- Yoneyama, T.; Takahashi, H.; Grudniewska, A.; Ban, S.; Umeyama, A.; Noji, M. Ergostane-Type Sterols from Several Cordyceps Strains. Nat. Prod. Commun. 2022, 17, 1934578X221105363. [Google Scholar] [CrossRef]
- Zhabinskii, V.N.; Drasar, P.; Khripach, V.A. Structure and biological activity of ergostane-type steroids from fungi. Molecules 2022, 27, 2103. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.; Wang, H. Pharmacological actions of Cordyceps, a prized folk medicine. J. Pharm. Pharmacol. 2005, 57, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-H.; Lin, C.-H.; Shih, C.-C. Ergostatrien-3β-ol from Antrodia camphorata inhibits diabetes and hyperlipidemia in high-fat-diet treated mice via regulation of hepatic related genes, glucose transporter 4, and AMP-activated protein kinase phosphorylation. J. Agric. Food Chem. 2015, 63, 2479–2489. [Google Scholar] [CrossRef]
- Huang, G.-J.; Huang, S.-S.; Lin, S.-S.; Shao, Y.-Y.; Chen, C.-C.; Hou, W.-C.; Kuo, Y.-H. Analgesic effects and the mechanisms of anti-inflammation of ergostatrien-3β-ol from Antrodia camphorata submerged whole broth in mice. J. Agric. Food Chem. 2010, 58, 7445–7452. [Google Scholar] [CrossRef]
- Liu, H.-P.; Kuo, Y.-H.; Cheng, J.; Chang, L.-Z.; Chang, M.-S.; Su, L.-W.; Chuang, T.-N.; Lin, W.-Y. Ergosta-7, 9 (11), 22-trien-3β-ol Rescues AD Deficits by Modulating Microglia Activation but Not Oxidative Stress. Molecules 2021, 26, 5338. [Google Scholar] [CrossRef]
- Konishi, H.; Kiyama, H. Microglial TREM2/DAP12 Signaling: A Double-Edged Sword in Neural Diseases. Front. Cell Neurosci. 2018, 12, 206. [Google Scholar] [CrossRef]
- Hsieh, W.-T.; Hsu, M.-H.; Lin, W.-J.; Xiao, Y.-C.; Lyu, P.-C.; Liu, Y.-C.; Lin, W.-Y.; Kuo, Y.-H.; Chung, J.-G. Ergosta-7, 9 (11), 22-trien-3β-ol Interferes with LPS Docking to LBP, CD14, and TLR4/MD-2 Co-Receptors to Attenuate the NF-κB Inflammatory Pathway In Vitro and Drosophila. Int. J. Mol. Sci. 2021, 22, 6511. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.M. Recombinant tissue plasminogen activator for the treatment of acute ischemic stroke. In Baylor University Medical Center Proceedings; Taylor & Francis: Abingdon, UK, 2011; Volume 24, pp. 257–259. [Google Scholar]
- Wang, Y.-H.; Chern, C.-M.; Liou, K.-T.; Kuo, Y.-H.; Shen, Y.-C. Ergostatrien-7, 9 (11), 22-trien-3β-ol from Antrodia camphorata ameliorates ischemic stroke brain injury via downregulation of p65NF-κ-B and caspase 3, and activation of Akt/GSK3/catenin-associated neurogenesis. Food Funct. 2019, 10, 4725–4738. [Google Scholar] [CrossRef] [PubMed]
- Juhaszova, M.; Zorov, D.B.; Kim, S.-H.; Pepe, S.; Fu, Q.; Fishbein, K.W.; Ziman, B.D.; Wang, S.; Ytrehus, K.; Antos, C.L. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Investig. 2004, 113, 1535–1549. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Jeong, S.-W.; Jung, K.-H.; Han, S.-Y.; Lee, S.-T.; Kim, M.; Roh, J.-K. Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J. Cereb. Blood Flow Metab. 2004, 24, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Poddar, R.; Thompson, J.; Rosenberg, G.; Yang, Y. Intranuclear matrix metalloproteinases promote DNA damage and apoptosis induced by oxygen–glucose deprivation in neurons. Neuroscience 2012, 220, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, P.-J.; Wang, M.-H.; Hsiao, C.-J.; Chen, C.-K.; Lin, F.-L.; Huang, S.-H.; Yen, J.-L.; Tsai, P.-H.; Kuo, Y.-H.; Hsiao, G. Ergosta-7,9(11),22-trien-3β-ol Alleviates Intracerebral Hemorrhage-Induced Brain Injury and BV-2 Microglial Activation. Molecules 2021, 26, 2970. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.M.; Cao, W.; Yao, K.W.; Liu, Z.Q.; Guo, J.Y. Anti-inflammation and antioxidant effect of Cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Metab. Brain Dis. 2012, 27, 159–165. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, Y.; Yan, Y.-b.; Lei, W.; Wu, Z.-x.; Liu, N.; Liu, S.; Shi, L.; Fan, Y. The protective effect of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, on diabetic osteopenia in alloxan-induced diabetic rats. Evid.-Based Complement. Altern. Med. 2013, 2013, 985636. [Google Scholar] [CrossRef]
- Qian, G.M.; Pan, G.F.; Guo, J.Y. Anti-inflammatory and antinociceptive effects of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis. Nat. Prod. Res. 2012, 26, 2358–2362. [Google Scholar] [CrossRef]
- Kuroda, S.; Siesjö, B.K. Reperfusion damage following focal ischemia: Pathophysiology and therapeutic windows. Clin. Neurosci. 1997, 4, 199–212. [Google Scholar]
- Xu, G.; Du, P.; An, L.; Lv, G.; Miao, L.; Guangxin, Y. Research progress of active polypeptides of Cordyceps militaris. In Proceedings of the 40th World Congress of Vine and Wine BIO Web of Conferences, Sofia, Bulgaria, 29 May–2 June 2017; p. 01002. [Google Scholar]
- Xie, L.; An, L.; Du, P. Study on the enzymolysis technology of Cordyceps militaris peptides and its effect on immune function in mice. Chin. Tradit. Pat. Med. 2016, 38, 2048–2050. [Google Scholar]
- Yuan, G.; An, L.; Sun, Y.; Xu, G.; Du, P. Improvement of Learning and Memory Induced by Cordyceps Polypeptide Treatment and the Underlying Mechanism. Evid.-Based Complement. Altern. Med. 2018, 2018, 9419264. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Inoue, K.; Yamamoto, S.; Ikumoto, T.; Sasaki, S.; Toyama, R.; Chiba, K.; Hoshino, Y.; Okumoto, T. Fungal metabolites. Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J. Antibiot. 1994, 47, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Kohara, T.; Nakao, N.; Arita, M.; Chiba, K.; Mishina, T.; Sasaki, S.; Fujita, T. Design, synthesis, and structure-activity relationships of 2-substituted-2-amino-1, 3-propanediols: Discovery of a novel immunosuppressant, FTY720. Bioorg. Med. Chem. Lett. 1995, 5, 853–856. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Suzuki, H.; Sozen, T.; Rolland, W.; Zhang, J.H. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke 2010, 41, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, Y.; Sugahara, K.; Kataoka, H.; Kawaguchi, T.; Masubuchi, Y.; Chiba, K. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. II. FTY720 prolongs skin allograft survival by decreasing T cell infiltration into grafts but not cytokine production in vivo. J. Immunol. 1998, 160, 5493–5499. [Google Scholar] [PubMed]
- Chiba, K. Discovery of fingolimod based on the chemical modification of a natural product from the fungus, Isaria sinclairii. J. Antibiot. 2020, 73, 666–678. [Google Scholar] [CrossRef]
- Jęśko, H.; Wencel, P.L.; Wójtowicz, S.; Strosznajder, J.; Lukiw, W.J.; Strosznajder, R.P. Fingolimod affects transcription of genes encoding enzymes of ceramide metabolism in animal model of Alzheimer’s disease. Mol. Neurobiol. 2020, 57, 2799–2811. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, X.; Yang, L.; Li, M.; Wood, K.; Liu, Q.; Zhu, X. Neuroprotective effects of fingolimod in mouse models of Parkinson’s disease. FASEB J. 2017, 31, 172–179. [Google Scholar] [CrossRef]
- Kappos, L.; Antel, J.; Comi, G.; Montalban, X.; O’Connor, P.; Polman, C.H.; Haas, T.; Korn, A.A.; Karlsson, G.; Radue, E.W. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl. J. Med. 2006, 355, 1124–1140. [Google Scholar] [CrossRef]
- FDA. FDA Approves First Oral Drug to Reduce MS Relapses; Press Release; FDA: Silver Spring, MD, USA, 2010. [Google Scholar]
- Zhu, X.Y.; Ma, T.T.; Li, Y.; Zhang, M.Q.; Zhao, L.; Liang, J.; Min, L.Q. Fingolimod protects against neurovascular unit injury in a rat model of focal cerebral ischemia/reperfusion injury. Neural Regen. Res. 2023, 18, 869–874. [Google Scholar] [PubMed]
- Lin, B.-Q.; Li, S.-P. Cordyceps as an herbal drug. In Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press: Boca Raton, FL, USA, 2011; Volume 5. [Google Scholar]
- Kwon, H.-W.; Shin, J.-H.; Lim, D.H.; Ok, W.J.; Nam, G.S.; Kim, M.J.; Kwon, H.-K.; Noh, J.-H.; Lee, J.-Y.; Kim, H.-H.; et al. Antiplatelet and antithrombotic effects of cordycepin-enriched WIB-801CE from Cordyceps militaris ex vivo, in vivo, and in vitro. BMC Complement. Altern. Med. 2016, 16, 508. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.C.; Wong, J.W.; Suen, Y.-K.; Kwok, T.; Fung, K.; Kong, S. Cordycepin induced eryptosis in mouse erythrocytes through a Ca 2+-dependent pathway without caspase-3 activation. Arch. Toxicol. 2007, 81, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Han, X.; Jin, H.; Ma, S. Arsenic species in Cordyceps sinensis and its potential health risks. Front. Pharmacol. 2019, 10, 1471. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Sun, Y.; Luo, F.; Wang, C. Bioactive Metabolites and Potential Mycotoxins Produced by Cordyceps Fungi: A Review of Safety. Toxins 2020, 12, 410. [Google Scholar] [CrossRef] [PubMed]
- Rodman, L.E.; Farnell, D.R.; Coyne, J.M.; Allan, P.W.; Hill, D.L.; Duncan, K.L.; Tomaszewski, J.E.; Smith, A.C.; Page, J.G. Toxicity of cordycepin in combination with the adenosine deaminase inhibitor 2’-deoxycoformycin in beagle dogs. Toxicol. Appl. Pharmacol. 1997, 147, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Pegram, R.A.; Wyatt, R.D. Avian gout caused by oosporein, a mycotoxin produced by Caetomium trilaterale. Poult Sci. 1981, 60, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Mallebrera, B.; Juan-Garcia, A.; Font, G.; Ruiz, M.J. Mechanisms of beauvericin toxicity and antioxidant cellular defense. Toxicol. Lett. 2016, 246, 28–34. [Google Scholar] [CrossRef]
- Jung, S.J.; Jung, E.S.; Choi, E.K.; Sin, H.S.; Ha, K.C.; Chae, S.W. Immunomodulatory effects of a mycelium extract of Cordyceps (Paecilomyces hepiali; CBG-CS-2): A randomized and double-blind clinical trial. BMC Complement. Altern. Med. 2019, 19, 77. [Google Scholar] [CrossRef]
- Chae, S.-W. Efficacy and Safety of Cordyceps Sinensis Mycelium Culture Extract(Paecilomyces Hepiali, CBG-CS-2) on Promotion of Immunity. 2019. Available online: https://clinicaltrials.gov/show/NCT02814617 (accessed on 25 August 2023).
- Jung, S.J.; Hwang, J.H.; Oh, M.R.; Chae, S.W. Effects of Cordyceps militaris supplementation on the immune response and upper respiratory infection in healthy adults: A randomized, double-blind, placebo-controlled study. J. Nutr. Health 2019, 52, 258–267. [Google Scholar] [CrossRef]
- Kang, H.J.; Baik, H.W.; Kim, S.J.; Lee, S.G.; Ahn, H.Y.; Park, J.S.; Park, S.J.; Jang, E.J.; Park, S.W.; Choi, J.Y. Cordyceps militaris enhances cell-mediated immunity in healthy Korean men. J. Med. Food 2015, 18, 1164–1172. [Google Scholar] [CrossRef]
- University of Oxford. Anti-Cancer Drug Derived from Fungus Shows Promise in Clinical Trials; University of Oxford: Oxford, UK, 2021. [Google Scholar]
- Xiao, Y.; Huang, X.-Z.; Zhu, J.-S. Randomized double-blind placebo-controlled clinical trial and assessment of fermentation product of Cordyceps sinensis (Cs-4) in enhancing aerobic capacity and respiratory function of the healthy elderly volunteers. Chin. J. Integr. Med. 2004, 10, 187–192. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, X.; Xiao, L.; Zhou, J.; Feng, L.; Wang, G. Efficacy and Safety of Cordyceps militaris as an Adjuvant to Duloxetine in the Treatment of Insomnia in Patients with Depression: A 6-Week Double-Blind, Randomized, Placebo-Controlled Trial. Front. Psychiatry 2021, 12, 754921. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-A.; Lin, T.-H.; Wang, J.-S.; Chen, J.-J.; Hsu, W.-K.; Ying, L.-C.; Liang, Z.-C. The effects of Cordyceps militaris fruiting bodies in micturition and prostate size in benign prostatic hyperplasia patients: A pilot study. Pharmacol. Res. Mod. Chin. Med. 2022, 4, 100143. [Google Scholar] [CrossRef]
- Heo, J.Y.; Baik, H.W.; Kim, H.J.; Lee, J.M.; Kim, H.W.; Choi, Y.S.; Won, J.H.; Hyun Mi Kim, W.I.P.; Kim, C.Y. The efficacy and safety of Cordyceps militaris in Korean adults who have mild liver dysfunction. J. Korean Soc. Parenter. Enter. Nutr. 2015, 7, 81–86. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Krochmal, R.; Abrazado, M.; Kim, W.; Cooper, C.B. Effect of Cs-4®(Cordyceps sinensis) on exercise performance in healthy older subjects: A double-blind, placebo-controlled trial. J. Altern. Complement. Med. 2010, 16, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, K.R.; Mock, M.G.; Roelofs, E.J.; Trexler, E.T.; Smith-Ryan, A.E. Chronic supplementation of a mushroom blend on oxygen kinetics, peak power, and time to exhaustion. J. Int. Soc. Sports Nutr. 2015, 12, 1. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, H.; Sharma, N.; An, S.S.A. Unique Bioactives from Zombie Fungus (Cordyceps) as Promising Multitargeted Neuroprotective Agents. Nutrients 2024, 16, 102. https://doi.org/10.3390/nu16010102
Sharma H, Sharma N, An SSA. Unique Bioactives from Zombie Fungus (Cordyceps) as Promising Multitargeted Neuroprotective Agents. Nutrients. 2024; 16(1):102. https://doi.org/10.3390/nu16010102
Chicago/Turabian StyleSharma, Himadri, Niti Sharma, and Seong Soo A. An. 2024. "Unique Bioactives from Zombie Fungus (Cordyceps) as Promising Multitargeted Neuroprotective Agents" Nutrients 16, no. 1: 102. https://doi.org/10.3390/nu16010102