BMI Increases in Individuals with COVID-19-Associated Olfactory Dysfunction
Abstract
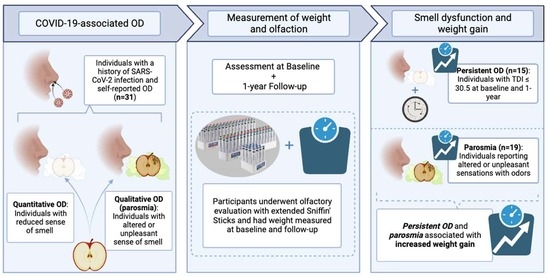
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khubchandani, J.; Price, J.H.; Sharma, S.; Wiblishauser, M.J.; Webb, F.J. COVID-19 pandemic and weight gain in American adults: A nationwide population-based study. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102392. [Google Scholar] [CrossRef] [PubMed]
- Saniasiaya, J.; Islam, A.; Abdullah, B. Prevalence of Olfactory Dysfunction in Coronavirus Disease 2019 (COVID-19): A Meta-analysis of 27,492 Patients. Laryngoscope 2021, 131, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.Y.; Wong, A.; Zhu, D.; Fastenberg, J.H.; Tham, T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol. Neck Surg. 2020, 163, 3–11. [Google Scholar] [CrossRef]
- Gary, J.B.; Gallagher, L.; Joseph, P.V.; Reed, D.; Gudis, D.A.; Overdevest, J.B. Qualitative Olfactory Dysfunction and COVID-19: An Evidence-Based Review with Recommendations for the Clinician. Am. J. Rhinol. Allergy 2023, 37, 95–101. [Google Scholar] [CrossRef]
- Ercoli, T.; Masala, C.; Pinna, I.; Orofino, G.; Solla, P.; Rocchi, L.; Defazio, G. Qualitative smell/taste disorders as sequelae of acute COVID-19. Neurol. Sci. 2021, 42, 4921–4926. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Masala, C.; Oleszkiewicz, A.; Englmaier, V.; Gunder, N.; Menzel, S.; Haehner, A.; Hummel, T. Nonlinear association between chemosensory dysfunction and body mass index. J. Sens. Stud. 2022, 37, e12715. [Google Scholar] [CrossRef]
- Wysokiński, A.; Sobów, T.; Kłoszewska, I.; Kostka, T. Mechanisms of the anorexia of aging—A review. AGE 2015, 37, 81. [Google Scholar] [CrossRef]
- Riera, C.E.; Tsaousidou, E.; Halloran, J.; Follett, P.; Hahn, O.; Pereira, M.M.; Ruud, L.E.; Alber, J.; Tharp, K.; Anderson, C.M.; et al. The Sense of Smell Impacts Metabolic Health and Obesity. Cell Metab. 2017, 26, 198–211.e5. [Google Scholar] [CrossRef]
- Velluzzi, F.; Deledda, A.; Onida, M.; Loviselli, A.; Crnjar, R.; Sollai, G. Relationship between Olfactory Function and BMI in Normal Weight Healthy Subjects and Patients with Overweight or Obesity. Nutrients 2022, 14, 1262. [Google Scholar] [CrossRef]
- Algahtani, S.N.; Alzarroug, A.F.; Alghamdi, H.K.; Algahtani, H.K.; Alsywina, N.B.; Bin Abdulrahman, K.A. Investigation on the Factors Associated with the Persistence of Anosmia and Ageusia in Saudi COVID-19 Patients. Int. J. Environ. Res. Public Health 2022, 19, 1047. [Google Scholar] [CrossRef]
- Perna, S.; Abdulsattar, S.; Alalwan, T.A.; Zahid, M.N.; Gasparri, C.; Peroni, G.; Faragli, A.; La Porta, E.; Redha, A.A.; Janahi, E.M.; et al. A cross-sectional analysis of post-acute COVID-19 symptoms. Ann. Ig. Med. Prev. Comunita 2022, 34, 478–489. [Google Scholar] [CrossRef]
- Bhutani, S.; Coppin, G.; Veldhuizen, M.G.; Parma, V.; Joseph, P.V. COVID-19 Related Chemosensory Changes in Individuals with Self-Reported Obesity. medRxiv 2021. preprint. [Google Scholar] [CrossRef] [PubMed]
- Taş, B.M.; Alpaydın, T.; Akçalı, S.; Kaygusuz, S.; Erol, Ö.Ö.; Şencan, Z.; Cömert, E.; Muluk, N.B.; Özel, G. Evaluation of Clinical Features and Olfactory Functions in COVID-19: A Multicentre Study. Cureus 2023, 15, e40027. [Google Scholar] [CrossRef] [PubMed]
- Budrewicz, S.; Zmarzly, A.; Raczka, D.; Szczepanska, A.; Koziorowska-Gawron, E.; Slotwinski, K.; Koszewicz, M. Clinical and nutritional correlations in Parkinson’s disease: Preliminary report. Adv. Clin. Exp. Med. 2019, 28, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Edland, S.D.; Cha, R.H.; Petersen, R.C.; Rocca, W.A. Incident dementia in women is preceded by weight loss by at least a decade. Neurology 2007, 69, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Fjaeldstad, A.W.; Smith, B. The Effects of Olfactory Loss and Parosmia on Food and Cooking Habits, Sensory Awareness, and Quality of Life—A Possible Avenue for Regaining Enjoyment of Food. Foods 2022, 11, 1686. [Google Scholar] [CrossRef]
- Tan, B.K.J.; Han, R.; Zhao, J.J.; Tan, N.K.W.; Quah, E.S.H.; Tan, C.J.-W.; Chan, Y.H.; Teo, N.W.Y.; Charn, T.C.; See, A.; et al. Prognosis and persistence of smell and taste dysfunction in patients with COVID-19: Meta-analysis with parametric cure modelling of recovery curves. BMJ 2022, 378, e069503. [Google Scholar] [CrossRef]
- Cooper, K.W.; Brann, D.H.; Farruggia, M.C.; Bhutani, S.; Pellegrino, R.; Tsukahara, T.; Weinreb, C.; Joseph, P.V.; Larson, E.D.; Parma, V.; et al. COVID-19 and the Chemical Senses: Supporting Players Take Center Stage. Neuron 2020, 107, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Mutiawati, E.; Fahriani, M.; Mamada, S.S.; Fajar, J.K.; Frediansyah, A.; Maliga, H.A.; Ilmawan, M.; Emran, T.B.; Ophinni, Y.; Ichsan, I.; et al. Anosmia and dysgeusia in SARS-CoV-2 infection: In-cidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms—A systematic review and meta-analysis. F1000Research 2021, 10, 40. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Hagen, M.M.; Butowt, R. Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis Reveals Significant Ethnic Differences. ACS Chem. Neurosci. 2020, 11, 2944–2961. [Google Scholar] [CrossRef]
- Landis, B.N.; Frasnelli, J.; Reden, J.; Lacroix, J.S.; Hummel, T. Differences Between Orthonasal and Retronasal Olfactory Functions in Patients with Loss of the Sense of Smell. Arch. Otolaryngol. Neck Surg. 2005, 131, 977–981. [Google Scholar] [CrossRef]
- Hintschich, C.A.; Niv, M.Y.; Hummel, T. The taste of the pandemic—Contemporary review on the current state of research on gustation in coronavirus disease 2019 (COVID-19). Int. Forum Allergy Rhinol. 2022, 12, 210–216. [Google Scholar] [CrossRef]
- Hannum, M.E.; Koch, R.J.; Ramirez, V.A.; Marks, S.S.; Toskala, A.K.; Herriman, R.D.; Lin, C.; Joseph, P.V.; Reed, D.R. Taste loss as a distinct symptom of COVID-19: A systematic review and meta-analysis. Chem. Senses 2022, 47, bjac001. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Albayay, J.; Höchenberger, R.; Bhutani, S.; Boesveldt, S.; Busch, N.A.; Croijmans, I.; Cooper, K.W.; de Groot, J.H.B.; Farruggia, M.C.; et al. COVID-19 affects taste independent of taste–smell confusions: Results from a combined chemosensory home test and online survey from a large global cohort. Chem. Senses 2023, 48, bjad020. [Google Scholar] [CrossRef]
- Doyle, M.E.; Appleton, A.; Liu, Q.-R.; Yao, Q.; Mazucanti, C.H.; Egan, J.M. Human Type II Taste Cells Express Angiotensin-Converting Enzyme 2 and Are Infected by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Am. J. Pathol. 2021, 191, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. BMI-related errors in the measurement of obesity. Int. J. Obes. 2008, 32, 56–59. [Google Scholar] [CrossRef]
- Jackson, A.S.; Ellis, K.J.; McFarlin, B.K.; Sailors, M.H.; Bray, M.S. Body mass index bias in defining obesity of diverse young adults: The Training Intervention and Genetics of Exercise Response (TIGER) Study. Br. J. Nutr. 2009, 102, 1084–1090. [Google Scholar] [CrossRef]
- Schlosser, R.; Smith, T.; Mace, J.; Alt, J.; Beswick, D.; Mattos, J.; Ramakrishnan, V.; Massey, C.; Soler, Z. The Olfactory Cleft Endoscopy Scale: A multi-institutional validation study in chronic rhinosinusitis. Rhinol. J. 2021, 59, 181–190. [Google Scholar] [CrossRef]
- Blumberg, S.J.; Bialostosky, K.; Hamilton, W.L.; Briefel, R.R. The effectiveness of a short form of the Household Food Security Scale. Am. J. Public Health 1999, 89, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Sekinger, B.; Wolf, S.; Pauli, E.; Kobal, G. ‘Sniffin’ Sticks’: Olfactory Performance Assessed by the Combined Testing of Odour Identification, Odor Discrimination and Olfactory Threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Oleszkiewicz, A.; Schriever, V.A.; Croy, I.; Hähner, A.; Hummel, T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Landis, B.N.; Frasnelli, J.; Croy, I.; Hummel, T. Evaluating the clinical usefulness of structured questions in parosmia assessment. Laryngoscope 2010, 120, 1707–1713. [Google Scholar] [CrossRef]
- Peng, M.; Coutts, D.; Wang, T.; Cakmak, Y.O. Systematic review of olfactory shifts related to obesity. Obes. Rev. 2019, 20, 325–338. [Google Scholar] [CrossRef]
- Watson, D.L.B.; Campbell, M.; Hopkins, C.; Smith, B.; Kelly, C.; Deary, V. Altered smell and taste: Anosmia, parosmia and the impact of long COVID-19. PLoS ONE 2021, 16, e0256998. [Google Scholar] [CrossRef]
- Liem, D.G.; Russell, C.G. The Influence of Taste Liking on the Consumption of Nutrient Rich and Nutrient Poor Foods. Front. Nutr. 2019, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Avesani, C.M. Pros and Cons of Body Mass Index as a Nutritional and Risk Assessment Tool in Dialysis Patients. Semin. Dial. 2015, 28, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Krems, C.; Heuer, T.; Hoffmann, I. Association between body mass index and macronutrients differs along the body mass index range of German adults: Results from the German National Nutrition Survey II. J. Nutr. Sci. 2021, 10, 8. [Google Scholar] [CrossRef]
- O’Dea, J.A.; Wilson, R. Socio-cognitive and nutritional factors associated with body mass index in children and adolescents: Possibilities for childhood obesity prevention. Health Educ. Res. 2006, 21, 796–805. [Google Scholar] [CrossRef]
- González-Monroy, C.; Gómez-Gómez, I.; Olarte-Sánchez, C.M.; Motrico, E. Eating Behaviour Changes during the COVID-19 Pandemic: A Systematic Review of Longitudinal Studies. Int. J. Environ. Res. Public Health 2021, 18, 11130. [Google Scholar] [CrossRef]
- Neira, C.; Godinho, R.; Rincón, F.; Mardones, R.; Pedroso, J. Consequences of the COVID-19 Syndemic for Nutritional Health: A Systematic Review. Nutrients 2021, 13, 1168. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, W.Y.; Sheridan, S.; Sit, C.H.-P.; Chen, X.-K.; Wong, S.H.-S. COVID-19 Pandemic Brings a Sedentary Lifestyle in Young Adults: A Cross-Sectional and Longitudinal Study. Int. J. Environ. Res. Public Health 2020, 17, 6035. [Google Scholar] [CrossRef] [PubMed]
- Abu Hatab, A.; Krautscheid, L.; Boqvist, S. COVID-19, Livestock Systems and Food Security in Developing Countries: A Systematic Review of an Emerging Literature. Pathogens 2021, 10, 586. [Google Scholar] [CrossRef]
- Aschenbrenner, K.; Hummel, C.; Teszmer, K.; Krone, F.; Ishimaru, T.; Seo, H.-S.; Hummel, T. The Influence of Olfactory Loss on Dietary Behaviors. Laryngoscope 2008, 118, 135–144. [Google Scholar] [CrossRef]
- Velluzzi, F.; Deledda, A.; Lombardo, M.; Fosci, M.; Crnjar, R.; Grossi, E.; Sollai, G. Application of Artificial Neural Networks (ANN) to Elucidate the Connections among Smell, Obesity with Related Metabolic Alterations, and Eating Habit in Patients with Weight Excess. Metabolites 2023, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Ferrulli, A.; Senesi, P.; Terruzzi, I.; Luzi, L. Eating Habits and Body Weight Changes Induced by Variation in Smell and Taste in Patients with Previous SARS-CoV-2 Infection. Nutrients 2022, 14, 5068. [Google Scholar] [CrossRef] [PubMed]
- Boesveldt, S.; Parma, V. The importance of the olfactory system in human well-being, through nutrition and social behavior. Cell Tissue Res. 2021, 383, 559–567. [Google Scholar] [CrossRef]
- Croy, I.; Nordin, S.; Hummel, T. Olfactory Disorders and Quality of Life—An Updated Review. Chem. Senses 2014, 39, 185–194. [Google Scholar] [CrossRef]
- Kohli, P.; Soler, Z.M.; Nguyen, S.A.; Muus, J.S.; Schlosser, R.J. The Association Between Olfaction and Depression: A Systematic Review. Chem. Senses 2016, 41, 479–486. [Google Scholar] [CrossRef]
- Altundag, A.; Yilmaz, E.; Kesimli, M.C. Modified Olfactory Training Is an Effective Treatment Method for COVID-19 Induced Parosmia. Laryngoscope 2022, 132, 1433–1438. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, S.W.; Basurrah, M.A.; Kim, D.H. The Efficacy of Olfactory Training as a Treatment for Olfactory Disorders Caused by Coronavirus Disease-2019: A Systematic Review and Meta-Analysis. Am. J. Rhinol. Allergy 2023, 37, 495–501. [Google Scholar] [CrossRef]
- Kershaw, J.C.; Mattes, R.D. Nutrition and taste and smell dysfunction. World J. Otorhinolaryngol.-Head Neck Surg. 2018, 4, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Parma, V.; Ohla, K.; Veldhuizen, M.G.; Niv, M.Y.; Kelly, C.E.; Bakke, A.J.; Cooper, K.W.; Bouysset, C.; Pirastu, N.; Dibattista, M.; et al. More Than Smell—COVID-19 Is Associated With Severe Impairment of Smell, Tastes, and Chemesthesis. Chem. Senses 2020, 45, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Raad, N.; Ghorbani, J.; Naeini, A.S.; Tajik, N.; Karimi-Galougahi, M. Parosmia in patients with COVID-19 and olfactory dysfunction. Int. Forum Allergy Rhinol. 2021, 11, 1497–1500. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | All (n = 31) | Quantitative Smell | Qualitative Smell | ||
|---|---|---|---|---|---|
| Persistent OD (n = 15) | Transient OD, Controls (n = 16) | Parosmia (n = 19) | No Parosmia, Controls (n = 12) | ||
| Age at Baseline (mean ± SD) | 44.850 ± 15.426 | 45.410 ± 16.251 | 44.324 ± 15.127 | 43.501 ± 13.724 | 46.985 ± 18.240 |
| Sex Assigned at Birth | |||||
| Female | 18 | 6 | 12 | 12 | 6 |
| Male | 13 | 9 | 4 | 7 | 6 |
| Race | |||||
| Asian | 3 | 1 | 2 | 1 | 2 |
| Black/African American | 2 | 1 | 1 | 1 | 1 |
| White | 22 | 12 | 10 | 13 | 9 |
| Unknown/ Declined to Answer | 4 | 1 | 3 | 4 | 0 |
| Ethnicity | |||||
| Hispanic/Latino | 4 | 1 | 3 | 4 | 0 |
| Non-Hispanic/Latino | 27 | 14 | 13 | 15 | 12 |
| Perceived COVID-19 Severity | |||||
| Substantially Less Severe | 12 | 6 | 6 | 6 | 6 |
| Slightly Less Severe | 8 | 3 | 5 | 4 | 4 |
| Same as Others | 5 | 1 | 4 | 4 | 1 |
| Slightly More Severe | 3 | 3 | 0 | 3 | 0 |
| Substantially More Severe | 1 | 1 | 0 | 1 | 0 |
| Unknown/Not Reported | 2 | 1 | 1 | 1 | 1 |
| Olfaction at Baseline (Mean ± SD) | Olfaction after 1 Year (Mean ± SD) | ΔBMI (kg/m2) | Test Statistic (Z) | Adjusted α | p-Value | |
|---|---|---|---|---|---|---|
| Persistent OD (n = 15) | ||||||
| TDI Score | 22.917 ± 5.948 | 24.400 ± 5.162 | 1.377 | −2.897 | 0.0125 | 0.004 * |
| Threshold | 4.050 ± 2.527 | 6.200 ± 2.910 | ||||
| Discrimination | 10.067 ± 2.658 | 9.733 ± 2.542 | ||||
| Identification | 8.800 ± 2.274 | 8.467 ± 2.029 | ||||
| Transient OD, controls (n = 16) | ||||||
| TDI Score | 29.875 ± 5.350 | 32.828 ± 3.625 | 0.650 | −1.758 | 0.05 | 0.079 |
| Threshold | 6.625 ± 2.676 | 9.891 ± 2.515 | ||||
| Discrimination | 12.188 ± 2.482 | 12.063 ± 2.144 | ||||
| Identification | 11.063 ± 2.516 | 10.875 ± 2.604 | ||||
| Parosmia (n = 19) | ||||||
| TDI Score | 25.421 ± 6.533 | 28.789 ± 4.534 | 1.082 | −2.535 | 0.0167 | 0.011 * |
| Threshold | 5.263 ± 2.933 | 7.789 ± 3.428 | ||||
| Discrimination | 10.947 ± 2.592 | 11.421 ± 2.317 | ||||
| Identification | 9.211 ± 2.573 | 9.579 ± 1.953 | ||||
| No parosmia, controls (n = 12) | ||||||
| TDI Score | 28.229 ± 6.560 | 28.688 ± 8.403 | 0.876 | −2.197 | 0.025 | 0.028 |
| Threshold | 5.563 ± 2.902 | 8.604 ± 3.176 | ||||
| Discrimination | 11.500 ± 3.060 | 10.167 ± 3.010 | ||||
| Identification | 11.167 ± 2.329 | 9.917 ± 3.554 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilarello, B.J.; Jacobson, P.T.; Tervo, J.P.; Gallagher, L.W.; Caruana, F.F.; Gary, J.B.; Saak, T.M.; Gudis, D.A.; Joseph, P.V.; Goldberg, T.E.; et al. BMI Increases in Individuals with COVID-19-Associated Olfactory Dysfunction. Nutrients 2023, 15, 4538. https://doi.org/10.3390/nu15214538
Vilarello BJ, Jacobson PT, Tervo JP, Gallagher LW, Caruana FF, Gary JB, Saak TM, Gudis DA, Joseph PV, Goldberg TE, et al. BMI Increases in Individuals with COVID-19-Associated Olfactory Dysfunction. Nutrients. 2023; 15(21):4538. https://doi.org/10.3390/nu15214538
Chicago/Turabian StyleVilarello, Brandon J., Patricia T. Jacobson, Jeremy P. Tervo, Liam W. Gallagher, Francesco F. Caruana, Joseph B. Gary, Tiana M. Saak, David A. Gudis, Paule V. Joseph, Terry E. Goldberg, and et al. 2023. "BMI Increases in Individuals with COVID-19-Associated Olfactory Dysfunction" Nutrients 15, no. 21: 4538. https://doi.org/10.3390/nu15214538