Euonymus alatus (Thunb.) Siebold Prevents Osteoclast Differentiation and Osteoporosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Osteoclast Precursor Cells
2.3. Cell Viability Assay
2.4. In Vitro Osteoclastogenesis Assay
2.5. In Vitro Osteoclast Function Assay
2.6. Western Blotting
2.7. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.8. OVX-Induced Osteoporosis Model
2.9. Micro-Computed Tomography (Micro-CT) Analysis
2.10. Ultrahigh Performance Liquid Chromatography–Tandem Mass Spectrometry (UHPLC-MS/MS) Analysis
2.11. Statistical Analysis
3. Results and Discussion
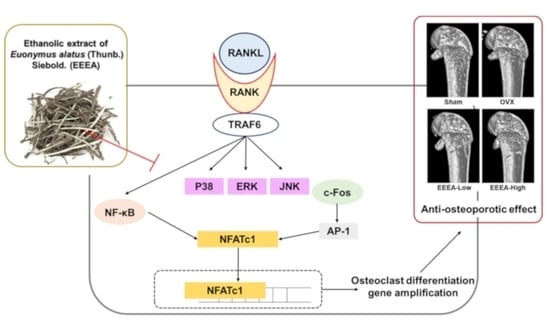
3.1. EEEA Inhibits Osteoclast Differentiation
3.2. EEEA Inhibits RANKL-Induced Signaling Pathways
3.3. EEEA Inhibits the Function of Mature Osteoclasts
3.4. EEEA Prevents Bone Loss in OVX Mice
3.5. Phytochemical Constituents of EEEA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sims, N.A.; Gooi, J.H. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin. Cell. Dev. Biol. 2008, 19, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Patil, S.; Xu, F.; Lin, X.; Qian, A. Role of Biomolecules in Osteoclasts and Their Therapeutic Potential for Osteoporosis. Biomolecules 2021, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Hak, D.J. The biology of fracture healing in osteoporosis and in the presence of anti-osteoporotic drugs. Injury 2018, 49, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Mbese, Z.; Aderibigbe, B.A. Bisphosphonate-Based Conjugates and Derivatives as Potential Therapeutic Agents in Osteoporosis, Bone Cancer and Metastatic Bone Cancer. Int. J. Mol. Sci. 2021, 22, 6869. [Google Scholar] [CrossRef] [PubMed]
- Nardone, V.; D’Asta, F.; Brandi, M.L. Pharmacological management of osteogenesis. Clinics 2014, 69, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Tella, S.H.; Gallagher, J.C. Biological agents in management of osteoporosis. Eur. J. Clin. Pharmacol. 2014, 70, 1291–1301. [Google Scholar] [CrossRef]
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. Int. J. Mol. Sci. 2019, 20, 2213. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, N.; Zheng, P.; Xu, X.; Liu, M.; Luo, D.; Xu, H.; Ju, D. Prevention and Treatment of Osteoporosis Using Chinese Medicinal Plants: Special Emphasis on Mechanisms of Immune Modulation. J. Immunol. Res. 2018, 2018, 6345857. [Google Scholar] [CrossRef]
- Lei, S.S.; Su, J.; Zhang, Y.; Huang, X.W.; Wang, X.P.; Huang, M.C.; Li, B.; Shou, D. Benefits and mechanisms of polysaccharides from Chinese medicinal herbs for anti-osteoporosis therapy: A review. Int. J. Biol. Macromol. 2021, 193, 1996–2005. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Signaling Pathways in Osteoclast Differentiation. Chonnam. Med. J. 2016, 52, 12–17. [Google Scholar] [CrossRef]
- Lee, K.; Seo, I.; Choi, M.H.; Jeong, D. Roles of Mitogen-Activated Protein Kinases in Osteoclast Biology. Int. J. Mol. Sci. 2018, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Kodama, J.; Kaito, T. Osteoclast Multinucleation: Review of Current Literature. Int. J. Mol. Sci. 2020, 21, 5685. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Nguyen, P.H.; Zhao, B.T.; Ali, M.Y.; Choi, J.S.; Min, B.S.; Woo, M.H. Chemical Constituents of Euonymus alatus (Thunb.) Sieb. and Their PTP1B and alpha-Glucosidase Inhibitory Activities. Phytother. Res. 2015, 29, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.; Baek, S.C.; Jo, M.S.; Kang, K.S.; Kim, K.H. (3beta,16alpha)-3,16-Dihydroxypregn-5-en-20-one from the Twigs of Euonymus alatus (Thunb.) Sieb. Exerts Anti-Inflammatory Effects in LPS-Stimulated RAW-264.7 Macrophages. Molecules 2019, 24, 3848. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.K.; Mun, J.; Seo, H.W.; Ryu, S.Y.; Kim, Y.S.; Lee, B.H.; Oh, K.S. Euonymus alatus extract attenuates LPS-induced NF-kappaB activation via IKKbeta inhibition in RAW 264.7 cells. J. Ethnopharmacol. 2011, 134, 288–293. [Google Scholar] [CrossRef]
- Jeong, E.J.; Yang, H.; Kim, S.H.; Kang, S.Y.; Sung, S.H.; Kim, Y.C. Inhibitory constituents of Euonymus alatus leaves and twigs on nitric oxide production in BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1394–1398. [Google Scholar] [CrossRef]
- Lee, S.; Moon, E.; Choi, S.U.; Kim, K.H. Lignans from the Twigs of Euonymus alatus (Thunb.) Siebold and Their Biological Evaluation. Chem. Biodivers 2016, 13, 1391–1396. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, C.; Ai, L.; Wang, L.; Li, L.; Fan, W.; Li, R.; He, L.; Wu, C.; Huang, Y. Traditional uses, botany, phytochemistry, pharmacology, separation and analysis technologies of Euonymus alatus (Thunb.) Siebold: A comprehensive review. J. Ethnopharmacol. 2020, 259, 112942. [Google Scholar] [CrossRef]
- Wang, Z.L.; Sun, H.H.; Liu, H.Y.; Ji, Q.X.; Niu, Y.T.; Ma, P.; Hao, G.; Zhang, J.X.; Yuan, Y.Y.; Chai, X.L.; et al. The water extracts of Euonymus alatus (Thunb.) Siebold attenuate diabetic retinopathy by mediating angiogenesis. J. Ethnopharmacol. 2022, 284, 114782. [Google Scholar] [CrossRef]
- Gu, D.R.; Yang, H.; Kim, S.C.; Hwang, Y.H.; Ha, H. Water Extract of Piper longum Linn Ameliorates Ovariectomy-Induced Bone Loss by Inhibiting Osteoclast Differentiation. Nutrients 2022, 14, 3667. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Lim, J.S.; Oh, J.; Lee, J.S.; Kim, J.S. Neuroprotective Effects of Euonymus alatus Extract on Scopolamine-Induced Memory Deficits in Mice. Antioxidants 2020, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Shrestha, R.; Lim, J.; Thapa Magar, T.B.; Kim, H.H.; Kim, Y.W. Euonymus alatus Twig Extract Protects against Scopolamine-Induced Changes in Brain and Brain-Derived Cells via Cholinergic and BDNF Pathways. Nutrients 2022, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Kim, B.S.; Lee, H.Y.; Park, Y.M.; Kim, Y.W.; Kim, M.J.; Yang, H.J.; Kim, M.S.; Bae, J.S. Euonymus alatus (Thunb.) Siebold leaf extract enhanced immunostimulatory effects in a cyclophosphamide-induced immunosuppressed rat model. Food Nutr. Res. 2023, 67. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, H.; Lan, Y.; Li, S.; Hashi, Y.; Chen, S. High-throughput and sensitive screening of compounds with deoxyribonucleic acid-binding activity by a high-performance liquid chromatography-tandem mass spectrometry-fluorescence detection technique using palmatine as a fluorescence probe. J. Chromatogr. A 2014, 1323, 123–134. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Ge, W.; Cai, G.; Guo, Y.; Gong, J. Spectrum-effect relationship between ultra-high-performance liquid chromatography fingerprints and antioxidant activities of Lophatherum gracile Brongn. Food Sci. Nutr. 2022, 10, 1592–1601. [Google Scholar] [CrossRef]
- Zhai, X.; Lenon, G.B.; Xue, C.C.; Li, C.G. Euonymus alatus: A Review on Its Phytochemistry and Antidiabetic Activity. Evid. Based Complement. Altern. Med. 2016, 2016, 9425714. [Google Scholar] [CrossRef]
- Ghiulai, R.; Mioc, M.; Racoviceanu, R.; Prodea, A.; Milan, A.; Coricovac, D.; Dehelean, C.; Avram, S.; Zamfir, A.D.; Munteanu, C.V.A.; et al. Structural Investigation of Betulinic Acid Plasma Metabolites by Tandem Mass Spectrometry. Molecules 2022, 27, 7359. [Google Scholar] [CrossRef]
- Choi, C.-I.; Lee, S.R.; Kim, K.H. Antioxidant and α-glucosidase inhibitory activities of constituents from Euonymus alatus twigs. Ind. Crops Prod. 2015, 76, 1055–1060. [Google Scholar] [CrossRef]
- Kang, H.R.; Eom, H.J.; Lee, S.R.; Choi, S.U.; Kang, K.S.; Lee, K.R.; Kim, K.H. Bioassay-guided Isolation of Antiproliferative Triterpenoids from Euonymus alatus Twigs. Nat. Prod. Commun. 2015, 10, 1929–1932. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef]
- Honma, M.; Ikebuchi, Y.; Kariya, Y.; Suzuki, H. Regulatory mechanisms of RANKL presentation to osteoclast precursors. Curr. Osteoporos. Rep. 2014, 12, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Rosenberg, E.; de Papp, A.E.; Duong, L.T. The osteoclast, bone remodelling and treatment of metabolic bone disease. Eur. J. Clin. Investig. 2012, 42, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef]
- Nakashima, T.; Takayanagi, H. New regulation mechanisms of osteoclast differentiation. Ann. N. Y. Acad. Sci. 2011, 1240, E13–E18. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in Osteoclast Differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Izawa, T.; Arakaki, R.; Mori, H.; Tsunematsu, T.; Kudo, Y.; Tanaka, E.; Ishimaru, N. The Nuclear Receptor AhR Controls Bone Homeostasis by Regulating Osteoclast Differentiation via the RANK/c-Fos Signaling Axis. J. Immunol. 2016, 197, 4639–4650. [Google Scholar] [CrossRef]
- Izawa, T.; Arakaki, R.; Ishimaru, N. Crosstalk between Cytokine RANKL and AhR Signalling in Osteoclasts Controls Bone Homeostasis. J. Cytokine Biol. 2017, 2, 1000114. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, Y.; Liu, Q.; Lan, Y.; Wei, C.; Tian, K.; Wu, L.; Lin, C.; Xu, J.; Zhao, J.; et al. Betulinic Acid Protects From Bone Loss in Ovariectomized Mice and Suppresses RANKL-Associated Osteoclastogenesis by Inhibiting the MAPK and NFATc1 Pathways. Front. Pharmacol. 2020, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.H.; Kwak, S.C.; Lee, M.S.; Yoon, K.H.; Kim, J.Y.; Lee, C.H. Betulinic Acid Inhibits RANKL-Induced Osteoclastogenesis via Attenuating Akt, NF-kappaB, and PLCgamma2-Ca(2+) Signaling and Prevents Inflammatory Bone Loss. J. Nat. Prod. 2020, 83, 1174–1182. [Google Scholar] [CrossRef]
- Zhao, D.; Li, X.; Zhao, Y.; Qiao, P.; Tang, D.; Chen, Y.; Xue, C.; Li, C.; Liu, S.; Wang, J.; et al. Oleanolic acid exerts bone protective effects in ovariectomized mice by inhibiting osteoclastogenesis. J. Pharmacol. Sci. 2018, 137, 76–85. [Google Scholar] [CrossRef]
- Zhao, D.; Shu, B.; Wang, C.; Zhao, Y.; Cheng, W.; Sha, N.; Li, C.; Wang, Q.; Lu, S.; Wang, Y. Oleanolic acid exerts inhibitory effects on the late stage of osteoclastogenesis and prevents bone loss in osteoprotegerin knockout mice. J. Cell. Biochem. 2020, 121, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.P.; Shi, L.Y.; Li, J.P.; Zeng, Y.; Liu, W.; Tang, S.Y.; Jia, L.J.; Zhang, J.; Gan, G.X. Oleanolic acid inhibits RANKL-induced osteoclastogenesis via ER alpha/miR-503/RANK signaling pathway in RAW264.7 cells. Biomed. Pharmacother. 2019, 117, 109045. [Google Scholar] [CrossRef]
- Fayed, H.A.; Barakat, B.M.; Elshaer, S.S.; Abdel-Naim, A.B.; Menze, E.T. Antiosteoporotic activities of isoquercitrin in ovariectomized rats: Role of inhibiting hypoxia inducible factor-1 alpha. Eur. J. Pharmacol. 2019, 865, 172785. [Google Scholar] [CrossRef]
- Ma, X.Q.; Han, T.; Zhang, X.; Wu, J.Z.; Rahman, K.; Qin, L.P.; Zheng, C.J. Kaempferitrin prevents bone lost in ovariectomized rats. Phytomedicine 2015, 22, 1159–1162. [Google Scholar] [CrossRef]
- Xing, L.Z.; Ni, H.J.; Wang, Y.L. Quercitrin attenuates osteoporosis in ovariectomized rats by regulating mitogen-activated protein kinase (MAPK) signaling pathways. Biomed. Pharmacother. 2017, 89, 1136–1141. [Google Scholar] [CrossRef]
- Satue, M.; Arriero Mdel, M.; Monjo, M.; Ramis, J.M. Quercitrin and taxifolin stimulate osteoblast differentiation in MC3T3-E1 cells and inhibit osteoclastogenesis in RAW 264.7 cells. Biochem. Pharmacol. 2013, 86, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M. Protective effect of quercitrin against hydrogen peroxide-induced dysfunction in osteoblastic MC3T3-E1 cells. Exp. Toxicol. Pathol. 2012, 64, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.H.; Yang, R.S.; Wang, K.C.; Lu, D.H.; Liou, H.C.; Ma, Y.; Chang, S.H.; Fu, W.M. Ethanol Extracts of Fresh Davallia formosana (WL1101) Inhibit Osteoclast Differentiation by Suppressing RANKL-Induced Nuclear Factor- kappa B Activation. Evid. Based Complement. Altern. Med. 2013, 2013, 647189. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, C.; Tian, B.; Liu, X.; Zhai, Z.; Qu, X.; Jiang, C.; Ouyang, Z.; Mao, Y.; Tang, T.; et al. The inhibition of RANKL-induced osteoclastogenesis through the suppression of p38 signaling pathway by naringenin and attenuation of titanium-particle-induced osteolysis. Int. J. Mol. Sci. 2014, 15, 21913–21934. [Google Scholar] [CrossRef]
- La, V.D.; Tanabe, S.; Grenier, D. Naringenin inhibits human osteoclastogenesis and osteoclastic bone resorption. J. Periodontal. Res. 2009, 44, 193–198. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Luo, M.; Shen, M.; Xu, C.; Xu, G.; Chen, Y.; Xia, L. Naringenin inhibits osteoclastogenesis through modulation of helper T cells-secreted IL-4. J. Cell. Biochem. 2018, 119, 2084–2093. [Google Scholar] [CrossRef]






| No | Retention Time (min) | Ion Mode | Error (ppm) | Formula | Expected Mass (m/z) | Measured Mass (m/z) | MS/MS Fragments (m/z) | Identifications |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.09 | 1.8547 | C15H14O6 | 289.0718 | 289.0723 | 289.0722, 245.0819, 109.028 | Catechin * | |
| 2 | 5.60 | 2.1714 | C15H14O6 | 289.0718 | 289.0724 | 289.0721, 245.0818, 203.0709, 125.0323 | Epicatechin * | |
| 3 | 5.83 | 2.5901 | C27H30O17 | 625.141 | 625.1426 | 439.6026, 421.9533, 300.0278, 151.0027 | Quercetin 3-O-sophoroside * | |
| 4 | 6.11 | 4.266 | C26H28O16 | 595.136 | 595.1319 | 300.0277, 271.0239, 224.6036, 151.0025 | Quercetin 3-O-sambubioside | |
| 5 | 6.72 | 2.561 | C21H20O12 | 463.0882 | 463.0894 | 303.0411, 300.0278, 299.0195, 272.0279 | Isoquercitrin | |
| 6 | 6.82 | 1.5343 | C27H30O14 | 577.1563 | 577.1572 | 431.1940, 285.0475 | Kaempferitrin * | |
| 7 | 7.47 | 2.4164 | C21H20O11 | 447.0933 | 447.0944 | 301.0347, 300.0269 | Quercitrin * | |
| 8 | 8.09 | 1.6359 | C15H12O6 | 287.0561 | 287.0566 | 287.0563, 259.0612, 243.0661, 125.023 | Dihydrokaempferol * | |
| 9 | 10.73 | 1.6436 | C15H12O5 | 271.0612 | 271.0616 | 271.0614, 151.0025, 119.0489, 93.0331 | Naringenin * | |
| 10 | 20.57 | 2.386 | C30H48O3 | 455.3531 | 455.3542 | 437.3282, 327.2552, 281.2494 | Betulinic acid * | |
| 11 | 20.79 | 2.6541 | C30H48O3 | 455.3531 | 455.3543 | 455.3535, 437.3281, 393.3023 | Oleanolic acid * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-J.; Jang, S.-A.; Kim, S.C.; Gu, D.R.; Yang, H.; Ryuk, J.A.; Ha, H. Euonymus alatus (Thunb.) Siebold Prevents Osteoclast Differentiation and Osteoporosis. Nutrients 2023, 15, 3996. https://doi.org/10.3390/nu15183996
Lee S-J, Jang S-A, Kim SC, Gu DR, Yang H, Ryuk JA, Ha H. Euonymus alatus (Thunb.) Siebold Prevents Osteoclast Differentiation and Osteoporosis. Nutrients. 2023; 15(18):3996. https://doi.org/10.3390/nu15183996
Chicago/Turabian StyleLee, Sung-Ju, Seon-A Jang, Seong Cheol Kim, Dong Ryun Gu, Hyun Yang, Jin Ah Ryuk, and Hyunil Ha. 2023. "Euonymus alatus (Thunb.) Siebold Prevents Osteoclast Differentiation and Osteoporosis" Nutrients 15, no. 18: 3996. https://doi.org/10.3390/nu15183996