Safety and Effects of Lactobacillus paracasei TISTR 2593 Supplementation on Improving Cholesterol Metabolism and Atherosclerosis-Related Parameters in Subjects with Hypercholesterolemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Abstract
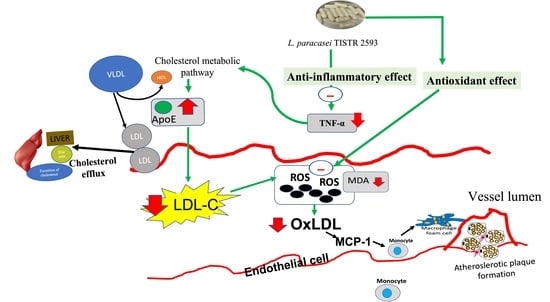
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design and Treatment
2.3. Blood Sampling and Biochemical Measurements
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Subjects
3.2. Effect of L. paracasei TISTR 2593 Supplementation on Blood Safety Parameters
3.3. Effect of L. paracasei TISTR 2593 Supplementation on Blood Lipid Profiles
3.4. Effect of L. paracasei TISTR 2593 Supplement on Oxidative Stress and Inflammation
3.5. Effect of L. paracasei TISTR 2593 Supplement on Adiponectin, apolipoprotein E, and Total Bile acid Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, R.D.; Gidding, S.S.; Hegele, R.A.; Cuchel, M.A.; Barter, P.J.; Watts, G.F.; Baum, S.J.; Catapano, A.L.; Chapman, M.J.; Defesche, J.C.; et al. International atherosclerosis society severe familial hypercholesterolemia panel. Defining severe familial hypercholesterolaemia and the implications for clinical management: A consensus statement from the international atherosclerosis society severe familial hypercholesterolemia panel. Lancet Diabetes Endocrinol. 2016, 4, 850–861. [Google Scholar] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kisugi, R. Mechanisms of LDL oxidation. Clin. Chim. Acta 2010, 411, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, P.; Tafelmeier, M.; Chittka, D.; Choi, S.H.; Zhang, L.; Byun, Y.S.; Almazan, F.; Yang, X.; Iqbal, N.; Chowdhury, P.; et al. MCP-1 binds to oxidized LDL and is carried by lipoprotein (a) in human plasma. J. Lipid. Res. 2013, 54, 1877–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.H.; Garruti, G.; Liu, M.; Portincasa, P.; Wang, D.Q. Cholesterol and lipoprotein metabolism and atherosclerosis: Recent advances in reverse cholesterol transport. Ann. Hepatol. 2018, 16, 27–42. [Google Scholar] [CrossRef]
- Charach, G.; Karniel, E.; Novikov, I.; Galin, L.; Vons, S.; Grosskopf, I.; Charach, L. Reduced bile acid excretion is an independent risk factor for stroke and mortality: A prospective follow-up study. Atherosclerosis 2020, 293, 79–85. [Google Scholar] [CrossRef]
- Sofat, R.; Cooper, J.A.; Kumari, M.; Casas, J.P.; Mitchell, J.P.; Acharya, J.; Thom, S.; Hughes, A.D.; Humphries, S.E.; Hingorani, A.D. Circulating apolipoprotein E concentration and cardiovascular disease risk: Meta-analysis of results from three studies. PLoS Med. 2016, 13, e1002146. [Google Scholar] [CrossRef] [Green Version]
- Christou, G.A.; Kiortsis, D.N. Adiponectin and lipoprotein metabolism. Obes. Rev. 2013, 14, 939–949. [Google Scholar] [CrossRef]
- Abuhammad, A. Cholesterol metabolism: A potential therapeutic target in Mycobacteria. Br. J. Clin. Pharmacol. 2017, 174, 2194–2208. [Google Scholar] [CrossRef]
- Millar, C.L.; Duclos, Q.; Blesso, C.N. Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Adv. Nutr. 2017, 8, 226–239. [Google Scholar] [CrossRef]
- Sun, Y.E.; Wang, W.; Qin, J. Anti-hyperlipidemia of garlic by reducing the level of total cholesterol and low-density lipoprotein: A meta-analysis. Medicine 2018, 97, e0255. [Google Scholar] [CrossRef] [PubMed]
- Lovati, M.R.; Manzoni, C.; Canavesi, A.; Sirtori, M.; Vaccarino, V.; Marchi, M.; Gaddi, G.; Sirtori, C.R. Soybean protein diet increases low density lipoprotein receptor activity in mononuclear cells from hypercholesterolemic patients. J. Clin. Investig. 1987, 80, 1498–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Kurpad, A.V.; Puri, S. Potential of probiotics in hypercholesterolemia: A meta-analysis. Indian J. Public Health. 2016, 60, 280. [Google Scholar] [PubMed]
- Kris-Etherton, P.M.; Zhao, G.; Binkoski, A.E.; Coval, S.M.; Etherton, T.D. The effects of nuts on coronary heart disease risk. Nutr. Rev. 2001, 59, 103–111. [Google Scholar] [CrossRef]
- Ding, E.L.; Hutfless, S.M.; Ding, X.; Girotra, S. Chocolate and prevention of cardiovascular disease: A systematic review. Nutr. Metab. 2006, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Fabian, E.; Elmadfa, I. Influence of daily consumption of probiotic and conventional yoghurt on the plasma lipid profile in young healthy women. Ann. Nutr. Metab. 2006, 50, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Razmpoosh, E.; Javadi, A.; Ejtahed, H.S.; Mirmiran, P.; Javadi, M.; Yousefinejad, A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: A randomized placebo controlled trial. Diabetes Metab. Syndr. Clin. Res. 2019, 13, 175–182. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Tirawat, Y.; Sivamaruthi, B.S.; Kesika, P.; Thangaleela, S.; Khongtan, S.; Khampithum, N.; Peerajan, S.; Chaiyasut, K.; Sirilun, S.; et al. Effect of Lactobacillus paracasei HII01 supplementation on total cholesterol, and on the parameters of lipid and carbohydrate metabolism, oxidative stress, inflammation and digestion in Thai hypercholesterolemic subjects. Appl. Sci. 2021, 11, 4333. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Chu, L.H.; Lee, C.L.; Pan, T.M. Atherosclerosis-preventing activity of lactic acid bacteria-fermented milk-soymilk supplemented with Momordica charantia. J. Agric. Food Chem. 2009, 57, 2065–2071. [Google Scholar] [CrossRef]
- Chiang, S.S.; Pan, T.M. Beneficial effects of Lactobacillus paracasei subsp. paracasei NTU 101 and its fermented products. Appl. Microbiol. Biotechnol. 2012, 93, 903–916. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [Green Version]
- Tarrah, A.; dos Santos Cruz, B.C.; Sousa Dias, R.; da Silva Duarte, V.; Pakroo, S.; Licursi de Oliveira, L.; Gouveia Peluzio, M.C.; Corich, V.; Giacomini, A.; Oliveira de Paula, S. Lactobacillus paracasei DTA81, a cholesterol-lowering strain having immunomodulatory activity, reveals gut microbiota regulation capability in BALB/c mice receiving high-fat diet. J. Appl. Microbiol. 2021, 131, 1942–1957. [Google Scholar] [CrossRef]
- London, L.E.; Kumar, A.H.; Wall, R.; Casey, P.G.; O’Sullivan, O.; Shanahan, F.; Hill, C.; Cotter, P.D.; Fitzgerald, G.F.; Ross, R.P.; et al. Exopolysaccharide-producing probiotic Lactobacilli reduce serum cholesterol and modify enteric microbiota in ApoE-deficient mice. J. Nutr. 2014, 144, 1956–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhutia, Y.; Ghosh, A.; Sherpa, M.L.; Pal, R.; Mohanta, P.K. Serum malondialdehyde level: Surrogate stress marker in the Sikkimese diabetics. J. Nat. Sci. Biol. Med. 2011, 2, 107. [Google Scholar] [PubMed] [Green Version]
- Tipple, T.E.; Rogers, L.K. Methods for the determination of plasma or tissue glutathione levels. Methods Mol. Biol. 2012, 889, 315–324. [Google Scholar]
- Zhong, B. How to calculate sample size in randomized controlled trial? J. Thorac. Dis. 2009, 1, 51–54. [Google Scholar]
- Gonnelli, S.; Caffarelli, C.; Stolakis, K.; Cuda, C.; Giordano, N.; Nuti, R. Efficacy and tolerability of a nutraceutical combination (Red Yeast Rice, Policosanols, and Berberine) in patients with low-moderate risk hypercholesterolemia: A double-Blind, placebo-controlled Study. Curr. Res. Clin. Exp. 2014, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chisolm, G.M.; Steinberg, D. The oxidative modification hypothesis of atherogenesis: An overview. Free Radic. Biol. Med. 2000, 28, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Markin, A.M.; Markina, Y.V.; Khotina, V.A.; Sukhorukov, V.N.; Postnov, A.Y.; Orekhov, A.N. Effects of pro-inflammatory cytokines on cholesterol accumulation in monocyte cell line culture. Atherosclerosis 2020, 315, e160. [Google Scholar] [CrossRef]
- Lamichhane, S.; Sen, P.; Alves, M.A.; Ribeiro, H.C.; Raunioniemi, P.; Hyötyläinen, T.; Orešič, M. Linking gut microbiome and lipid metabolism: Moving beyond associations. Metabolites 2021, 11, 55. [Google Scholar] [CrossRef]
- Falcinelli, S.; Rodiles, A.; Hatef, A.; Picchietti, S.; Cossignani, L.; Merrifield, D.L.; Unniappan, S.; Carnevali, O. Influence of probiotics administration on gut microbiota core: A review on the effects on appetite control, glucose, and lipid metabolism. J. Clin. Gastroenterol. 2018, 52, S50–S56. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Q.; Ren, Y.; Ruan, Z. Effect of probiotic Lactobacillus on lipid profile: A systematic review and meta-analysis of randomized, controlled trials. PLoS ONE 2017, 12, e0178868. [Google Scholar] [CrossRef]
- Naseri, K.; Saadati, S.; Ghaemi, F.; Ashtary-Larky, D.; Asbaghi, O.; Sadeghi, A.; Afrisham, R.; de Courten, B. The effects of probiotic and synbiotic supplementation on inflammation, oxidative stress, and circulating adiponectin and leptin concentration in subjects with prediabetes and type 2 diabetes mellitus: A GRADE-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials. Eur. J. Nutr. 2022, 14, 1–19. [Google Scholar]
- Vincenzi, A.; Goettert, M.I.; de Souza, C.F.V. An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-α) signaling and gene expression. Cytokine Growth Factor Rev. 2021, 57, 27–38. [Google Scholar] [CrossRef]
- Memon, R.A.; Grunfeld, C.A.R.L.; Moser, A.H.; Feingold, K.R. Tumor necrosis factor mediates the effects of endotoxin on cholesterol and triglyceride metabolism in mice. Endocrinology 1993, 132, 2246–2253. [Google Scholar] [CrossRef]
- Olejniczak-Staruch, I.; Narbutt, J.; Ceryn, J.; Skibińska, M.; Bednarski, I.; Woźniacka, A.; Sieniawska, J.; Kraska-Gacka, M.; Ciążyńska, M.; Śmigielski, J.; et al. AntiTNF-alpha therapy normalizes levels of lipids and adipokines in psoriatic patients in the real-life settings. Sci. Rep. 2021, 11, 9289. [Google Scholar] [CrossRef]
- Rasmussen, K.L.; Tybjærg-Hansen, A.; Nordestgaard, B.G.; Frikke-Schmidt, R. Plasma levels of apolipoprotein E and risk of ischemic heart disease in the general population. Atherosclerosis 2016, 246, 63–70. [Google Scholar] [CrossRef]
- Starck, M.; Bertrand, P.; Pépin, S.; Schiele, F.; Siest, G.; Galteau, M.M. Effects of pro-inflammatory cytokines on apolipoprotein E secretion by a human astrocytoma cell line (CCF-STTG1). Cell Biochem. Funct. 2000, 18, 9–16. [Google Scholar] [CrossRef]
- Yue, L.; Rasouli, N.; Ranganathan, G.; Kern, P.A.; Mazzone, T. Divergent effects of peroxisome proliferator-activated receptor γ agonists and tumor necrosis factor α on adipocyte ApoE expression. J. Biol. Chem. 2004, 279, 47626–47632. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, L.M.; Wu, J. Cross-talk between apolipoprotein E and cytokines. Mediat. Inflamm 2011, 2011, 949072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csonka, C.; Sárközy, M.; Pipicz, M.; Dux, L.; Csont, T. Modulation of hypercholesterolemia-induced oxidative/nitrative stress in the heart. Oxid. Med. Cell Longev. 2016, 2016, 3863726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lykkesfeldt, J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin. Chim. Acta 2007, 380, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Pirinccioglu, A.G.; Gökalp, D.; Pirinccioglu, M.; Kizil, G.; Kizil, M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin. Biochem. 2010, 43, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Pourrajab, B.; Fatahi, S.; Sohouli, M.H.; Găman, M.A.; Shidfar, F. The effects of probiotic/synbiotic supplementation compared to placebo on biomarkers of oxidative stress in adults: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 490–507. [Google Scholar] [CrossRef] [PubMed]
- Kleniewska, P.; Pawliczak, R. Influence of synbiotics on selected oxidative stress parameters. Oxid. Med. Cell Longev. 2017, 2017, 9315375. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.; Kleniewska, P.; Pawliczak, R. Antioxidative activity of probiotics. Arch. Med. Sci. 2019, 17, 792–804. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef] [Green Version]
- Rouhani, M.H.; Hadi, A.; Ghaedi, E.; Salehi, M.; Mahdavi, A.; Mohammadi, H. Do probiotics, prebiotics and synbiotics affect adiponectin and leptin in adults? A systematic review and meta-analysis of clinical trials. Clin. Nutr. 2019, 38, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.; Conceição, L.; Rosa, D.; Siqueira, N.; Peluzio, M. Mechanisms responsible for the hypocholesterolemia effect of regular consumption of probiotics. Nutr. Res. Rev. 2017, 30, 36–49. [Google Scholar] [CrossRef]
- Culpepper, T.; Rowe, C.C.; Rusch, C.T.; Burns, A.M.; Federico, A.P.; Girard, S.A.; Tompkins, T.A.; Nieves, C., Jr.; Dennis-Wall, J.C.; Christman, M.C.; et al. Three probiotic strains exert different effects on plasma bile acid profiles in healthy obese adults: Randomised, double-blind placebo-controlled crossover study. Benef. Microbes 2019, 10, 497–509. [Google Scholar] [CrossRef]

| Parameters | L. paracasei TISTR 2593 | p-Value within Group | Placebo | p-Value within Group | p-Value between Groups | ||
|---|---|---|---|---|---|---|---|
| Baseline | 90-Day | Baseline | 90-Day | ||||
| Body mass index (kg/m2) | 26.21 ± 3.82 | 25.31 ± 3.33 | 0.709 | 25.11 ± 4.24 | 25.79 ± 6.10 | 0.898 | 0.750 |
| Systolic blood pressure (mmHg) | 124.43 ± 19.19 | 123.14 ± 19.18 | 0.284 | 117.39 ± 29.19 | 125.95 ± 23.42 | 0.368 | 0.665 |
| Diastolic blood pressure (mmHg) | 78.43 ± 11.65 | 77.77 ± 10.67 | 0.771 | 80.61 ± 15.13 | 79.95 ± 13.55 | 0.987 | 0.967 |
| Heart rate (Bmp) | 78.43 ± 14.45 | 75.91 ± 11.73 | 0.787 | 81.78 ± 13.21 | 79.55 ± 11.28 | 0.737 | 0.785 |
| Calorie intake (Kcal) | 2771.38 ± 476.77 | 2558.12 ± 698.80 | 0.976 | 2670.85 ± 533.06 | 2645.38 ± 792.61 | 0.992 | 0.967 |
| Blood Parameters | L. paracasei TISTR 2593 | p-Value within Group | Placebo | p-Value within Group | p-Value between Groups | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Mean ± SD | 45-Day Mean ± SD Mean Difference (95% CI) from Baseline | 90-Day Mean ± SD Mean Difference (95% CI) from Baseline | Baseline Mean ± SD | 45-Day Mean ± SD Mean Difference (95% CI) from Baseline | 90-Day Mean ± SD Mean Difference (95% CI) from Baseline | ||||
| FBG (mg/dL) | 95.82 ± 8.13 | 97.14 ± 6.37 1.32 (−10.75 to−1.89) | 96.70 ± 8.40 0.88 (−10.37 to −1.40) | 0.189 | 96.09 ± 15.78 | 99.26 ± 13.20 3.17 (−13.94 to 7.12) | 101.50 ± 22.61 5.41 (−15.70 to 5.89) | 0.646 | 0.133 |
| BUN (mg/dL) | 12.48 ± 2.51 | 10.85 ± 3.84 −1.63 (−3.50 to 16.04) | 12.45 ± 2.80 −0.03 (−1.64 to 2.19) | 0.381 | 12.43 ± 2.41 | 12.51 ± 2.36 0.08 (−4.25 to 16.79) | 13.54 ± 2.89 1.11 (−1.64 to 2.19) | 0.238 | 0.772 |
| Creatinine (mg/dL) | 0.70 ± 0.09 | 0.69 ± 0.11 −0.01 (−0.11 to 0.03) | 0.66 ± 0.66 −0.04 (−0.09 to 0.09) | 0.605 | 0.73 ± 0.14 | 0.68 ± 0.14 −0.05 (−0.11 to 0.03) | 0.69 ± 0.15 −0.04 (−0.09 to 0.09) | 0.473 | 0.972 |
| AST (U/L) | 22.71 ± 10.34 | 21.62 ± 8.47 −1.09 (−43.57 to 1.93) | 23.00 ± 7.31 0.29 (−57.20 to3.18) | 0.170 | 25.47 ± 13.96 | 25.37 ±11.48 0.12 (−43.12 to 1.49) | 24.78 ± 7.22 −0.69 (−58.90 to 4.89) | 0.480 | 0.193 |
| ALT (U/L) | 25.85 ± 418.66 | 26.95 ± 24.06 1.12 (−73.63 to −6.14) | 28.00 ± 19.29 2.15 (−73.64 to −3.32) | 0.531 | 28.72 ± 13.30 | 27.28 ± 13.06 −1.44 (−72.97 to −6.80) | 30.76 ± 16.67 2.04 (−75.36 to −1.60) | 0.278 | 0.142 |
| ALP (U/L) | 53.91 ± 6.28 | 56.70 ± 12.72 2.79 (−18.09 to 9.09) | 56.81 ± 11.73 2.91 (−19.80 to 9.14) | 0.279 | 62.80 ± 24.16 | 61.33± 13.67 −1.47 (−17.90 to 8.90) | 60.19 ± 12.93 −2.61 (−20.25 to 9.59) | 0.314 | 0.461 |
| Sodium (mmol/L) | 139.36 ± 0.92 | 138.61 ± 1.44 −0.75 (−0.97 to 1.09) | 139.10 ± 1.64 −0.26 (−0.56 to 1.47) | 0.308 | 140.54 ± 1.94 | 138.99 ± 1.92 −1.55 (−0.99 to 1.11) | 139.57 ± 1.69 −0.97 (−0.56 to 1.47) | 0.519 | 0.376 |
| Potassium (mmol/L) | 4.29 ± 0.40 | 4.32 ± 0.31 0.03 (−0.24 to 0.09) | 4.15 ± 0.29 −0.14 (−0.31 to 0.06) | 0.900 | 4.22 ± 0.30 | 4.25 ± 0.23 0.03 (−0.24 to 0.09) | 4.16 ± 0.30 −0.06 (−0.31 to 0.06) | 0.514 | 0.179 |
| Chloride (mmol/L) | 105.82 ± 1.99 | 103.91 ± 3.09 −1.91 (−0.94 to 2.06) | 104.10 ± 2.84 −1.72 (−0.11 to 2.65) | 0.166 | 105.54 ± 1.76 | 104.41 ± 1.50 −1.13 (−0.91 to 2.03) | 105.29 ± 1.42 −0.25 (−0.15 to 2.68) | 0.453 | 0.718 |
| Hemoglobin (g/dL) | 13.22 ± 1.357 | 13.03 ± 1.40 −0.19 (−0.64 to1.27) | 13.32 ± 1.56 −0.1 (−1.13 to 0.84) | 0.614 | 13.19 ± 1.26 | 13.08 ± 1.35 −0.11 (−0.63 to 1.26) | 13.57 ± 1.70 0.38 (−1.14 to 0.85) | 0.145 | 0.227 |
| Hematocrit (%) | 39.40 ± 4.147 | 39.55 ± 4.39 0.15 (−3.74 to 1.91) | 39.85 ± 4.12 0.45 (−2.13 to 4.04) | 0.939 | 42.00 ± 5.09 | 40.85 ± 5.12 −1.15 (−3.70 to 1.87) | 40.84 ± 5.08 −1.16 (−2.17 to 4.08) | 0.757 | 0.430 |
| WBC counts (cell/cu.mm) | 6395 ± 1775.70 | 7130 ± 2237.97 735 (−1689.10 to 865.08) | 6955 ± 2711.86 560 (−238.86 to 2830.63) | 0.566 | 7595 ± 1990.63 | 6965 ± 1967.97 −630 (−1669.13 to 845.11) | 7236.84 ± 1990.87 −358.16 (−293.53 to 2885.30) | 0.582 | 0.722 |
| Neutrophil (%) | 59.15 ± 5.76 | 60.6 ± 7.68 1.50 (−5.64 to 2.91) | 60.25 ± 9.71 1.10 (−0.48 to 8.70) | 0.585 | 62.40 ± 5.47 | 59.15 ± 7.07 −3.25 (−5.66 to 2.92) | 60.37 ± 6.31 −2.03 (−0.46 to 8.68) | 0.249 | 0.311 |
| Lymphocyte (%) | 36.45 ± 7.997 | 34.30 ± 7.97 −2.15 (−2.76 to 5.84) | 34.45 ± 9.52 −2.01 (−8.81 to 0.25) | 0.706 | 34.90 ± 5.73 | 35.2 ± 6.83 2.39 (−2.82 to 5.89) | 33.84 ± 6.16 5.30 (−8.79 to 0.23) | 0.232 | 0.592 |
| Monocyte (%) | 3.60 ± 1.095 | 3.15 ± 0.93 0.00 (−0.81 to 0.55) | 3.35 ± 0.88 −0.25 (−0.63 to 0.97) | 0.316 | 3.70 ± 1.13 | 3.65 ± 1.31 −0.17 (−0.80 to 0.55) | 3.79 ± 1.23 0.74 (−0.64 to 0.98) | 0.932 | 0.412 |
| Eosinophil (%) | 2.00 ± 0.00 | 2.00 ± 0.00 0.0 (-) | 2.00 ± 0.00 0.0 (-) | 1.000 | 2.00 ± 0.00 | 2.00 ± 0.00 0.0 (-) | 2 ± 0.00 0 (-) | 1.000 | 1.000 |
| Platelet counts (cell/cu.mm) | 266,575 ± 89,903.64 | 290,050 ± 70,576.93 23,475 (−61,352.59 to 12,536.85) | 279,900 ± 87,735.19 13,325 (−13,678.20 to 71,760.45) | 0.647 | 263,040 ± 919,78.48 | 279,130 ± 92,468.04 16,090 (−60,706.21 to 11,890.48) | 286,947 ± 63,605.97 23,907 (−14,090.77 to 72,173.02) | 0.648 | 0.430 |
| MCV (fL) | 80.44 ± 6.988 | 80.62 ± 7.02 0.17 (−0.50 to 7.77) | 80.46 ± 5.65 0.02 (−9.58 to 0.35) | 0.996 | 78.39 ± 8.99 | 79.77 ± 7.37 1.38 (−0.51 to 7.78) | 76.31 ± 8.52 −2.90 (−9.63 to 0.40) | 0.716 | 0.510 |
| MCH (pg) | 26.74 ± 2.93 | 26.34 ± 3.27 −0.40 (−0.55 to 3.55) | 26.96 ± 3.07 0.22 (−34.92 to 9.26) | 0.798 | 26.11 ± 3.36 | 25.07 ± 2.92 −1.04 (−0.55 to 3.55) | 25.58 ± 4.16 −0.53 (−35.03 to 9.37) | 0.573 | 0.498 |
| MCHC (g/dL) | 33.53 ± 1.139 | 32.74 ± 1.66 −0.79 (−0.13 to 1.91) | 33.48 ± 1.86 −0.05 (−2.03 to 0.02) | 0.877 | 32.14 ± 1.45 | 31.08 ± 1.37 −1.06 (−0.13 to 1.91) | 33.39 ± 2.27 1.25 (−2.03 to 0.03) | 0.270 | 0.913 |
| Lipid Profiles | L. paracasei TISTR 2593 | Placebo | p-Value between Groups | ||||
|---|---|---|---|---|---|---|---|
| Baseline Mean ± SD | 45-Day Mean ± SD Mean Difference (95% CI) from Baseline | 90-Day Mean ± SD Mean Difference (95% CI) from Baseline | Baseline Mean ± SD | 45-Day Mean ± SD Mean Difference (95% CI) from Baseline | 90-Day Mean ± SD Mean Difference (95% CI) from Baseline | ||
| Total cholesterol (mg/dL) | 233.50 ± 41.59 | 224.90 ± 34.41 −8.60 (−27.68 to 21.98) | 227.75 ± 33.45 −5.75 (−36.28 to 1 3.38) | 231.48 ± 40.51 | 252.13 ± 47.46 20.65 (−46.01 to 4.71) | 246.70 ± 40.42 15.22 (−41.52 to 11.07) | 0.072 |
| Triglyceride (mg/dL) | 143.50 ± 40.05 | 153.60 ± 44.90 10.10 (−32.90 to 38.37) | 151.21 ± 41.42 7.71 (−23.17 to 48.10) | 146.09 ± 55.74 | 157.96 ± 61.24 11.87 (−47.99 to 24.25) | 157.14 ± 66.99 11.06 (−48.03 to 25.92) | 0.080 |
| HDL-C (mg/dL) | 53.35 ± 10.71 | 56.75 ± 8.98 3.40 (−7.27 to 9.07) | 55.85 ± 10.83 2.50 (−3.87 to 12.47) | 52.48 ± 10.65 | 58.65 ± 14.95 6.17 (−13.46 to 1.11) | 57.00 ± 10.90 4.52 (−11.98 to 2.94) | 0.125 |
| LDL-C (mg/dL) | 155.15 ± 33.03 | 137.40 ± 32.44 * −17.75 (−23.84 to 18.94) | 137.63 ± 29.24 * −17.52 (−41.59 to 1.19) | 151.61 ± 36.13 | 161.87 ± 36.98 10.26 (−32.39 to 11.87) | 163.00 ± 39.94 11.39 (−35.01 to 12.23) | 0.004 + |
| Parameters | L. paracasei TISTR 2593 | Placebo | p-Value between Groups | ||||
|---|---|---|---|---|---|---|---|
| Baseline Mean ± SD | 45-Day Mean ± SD Mean Difference (95% CI) from Baseline | 90-Day Mean ± SD Mean Difference (95% CI) from Baseline | Baseline Mean ± SD | 45-Day Mean ± SD Mean Difference (95% CI) from Baseline | 90-Day Mean ± SD Mean Difference (95% CI) from Baseline | ||
| MDA (nmol/mL) | 0.069 ± 0.04 | 0.045 ± 0.02 * −0.03 (0.01 to 0.04) | 0.030 ± 0.01 *** −0.04 (0.02 to 0.06) | 0.071 ± 0.03 | 0.064 ± 0.01 −0.01 (−0.07 to −0.02) | 0.078 ± 0.01 0.01 (−0.02 to 0.06) | 0.000 +++ |
| GSH (µg/mL) | 3.521 ± 1.94 | 4.031 ± 1.33 0.51 (−1.40 to 0.39) | 3.387 ± 0.53 −0.14 (−0.78 to 1.05) | 3.561 ± 1.46 | 3.844 ± 1.93 0.28 (−1.21 to 0.64) | 3.337 ± 0.46 −0.23 (−0.70 to 1.15) | 0.150 |
| TNF-α (pg/mL) | 11.163 ± 6.87 | 9.656 ± 1.74 −1.51 (−1.61 to 4.62) | 6.063 ± 2.79 ** −5.10 (1.90 to 8.30) | 11.541 ± 7.39 | 12.882 ± 2.57 1.34 (−4.37 to 1.81) | 9.759 ± 4.50 −1.78 (−1.29 to 4.98) | 0.024 + |
| IL-6 (pg/mL) | 1.207 ± 0.39 | 1.186 ± 0.21 −0.02 (−0.15 to 0.19) | 1.052 ± 0.11 −0.16 (0.01 to 0.33) | 1.325 ± 0.94 | 1.463 ± 0.43 0.14 (−0.54 to 0.27) | 1.218 ± 0.29 −0.11 (−0.22 to 0.54) | 0.134 |
| IL-10 (pg/mL) | 4.361 ± 2.52 | 5.138 ± 3.71 0.78 (−2.68 to 1.12) | 3.763 ± 2.03 −0.60 (−1.46 to 2.66) | 4.357 ± 2.55 | 4.214 ± 4.03 −0.14 (−1.98 to 2.27) | 3.877 ± 2.364 −0.480 (−1.08 to 2.84) | 0.659 |
| MCP-1 (pg/mL) | 1.942 ± 1.19 | 1.788 ± 1.22 −0.10 (−0.60 to 0.80) | 1.800 ± 1.01−0.09 (−0.65 to 0.83) | 1.890 ± 1.94 | 1.823 ± 1.06 −0.12 (−0.58 to 0.81) | 2.037 ± 1.37 0.10 (−0.81 to 0.62) | 0.360 |
| Adiponectin (ng/mL) | 37.199 ± 0.57 | 35.132 ± 0.60 *** −2.07 (1.45 to 2.68) | 35.251 ± 1.47 *** −3.95 (3.33 to 4.56) | 37.523 ± 0.44 | 32.627 ± 0.55 *** −1.90 (1.36 to 2.43) | 32.768 ± 1.96 *** −5.76 (5.20 to 6.31) | 0.005 ++ |
| APOE (ng/mL) | 0.751 ± 0.09 | 0.872 ± 0.16 * 0.12 (−0.21 to −0.03) | 0.887 ± 0.12 ** 0.14 (−0.23 to −0.04) | 0.783 ± 0.09 | 0.817 ± 0.10 0.03 (−0.10 to 0.03) | 0.806 ± 0.06 0.02 (−0.09 to 0.04) | 0.088 |
| TBA (µmol/L) | 4.560 ± 3.13 | 4.214 ± 3.03 −0.35 (−2.24 to 2.93) | 5.715 ± 4.89 1.16 (−3.66 to 1.35) | 4.619 ± 3.02 | 5.701 ± 6.15 1.08 (−4.41 to 2.25) | 5.983 ± 5.04 1.36 (−4.70 to 1.96) | 0.100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khongrum, J.; Yingthongchai, P.; Boonyapranai, K.; Wongtanasarasin, W.; Aobchecy, P.; Tateing, S.; Prachansuwan, A.; Sitdhipol, J.; Niwasabutra, K.; Thaveethaptaikul, P.; et al. Safety and Effects of Lactobacillus paracasei TISTR 2593 Supplementation on Improving Cholesterol Metabolism and Atherosclerosis-Related Parameters in Subjects with Hypercholesterolemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 661. https://doi.org/10.3390/nu15030661
Khongrum J, Yingthongchai P, Boonyapranai K, Wongtanasarasin W, Aobchecy P, Tateing S, Prachansuwan A, Sitdhipol J, Niwasabutra K, Thaveethaptaikul P, et al. Safety and Effects of Lactobacillus paracasei TISTR 2593 Supplementation on Improving Cholesterol Metabolism and Atherosclerosis-Related Parameters in Subjects with Hypercholesterolemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 2023; 15(3):661. https://doi.org/10.3390/nu15030661
Chicago/Turabian StyleKhongrum, Jurairat, Pratoomporn Yingthongchai, Kongsak Boonyapranai, Wachira Wongtanasarasin, Paitoon Aobchecy, Suriya Tateing, Aree Prachansuwan, Jaruwan Sitdhipol, Kanidta Niwasabutra, Punnathon Thaveethaptaikul, and et al. 2023. "Safety and Effects of Lactobacillus paracasei TISTR 2593 Supplementation on Improving Cholesterol Metabolism and Atherosclerosis-Related Parameters in Subjects with Hypercholesterolemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial" Nutrients 15, no. 3: 661. https://doi.org/10.3390/nu15030661