Vitamin D Status in Adolescents during COVID-19 Pandemic: A Cross-Sectional Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, M.; Dantas Damascena, A.; Galvão Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef]
- Bleakley, A.S.; Licciardi, P.V.; Binks, M.J. Vitamin D Modulation of the Innate Immune Response to Paediatric Respiratory Pathogens Associated with Acute Lower Respiratory Infections. Nutrients 2021, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- de Winter, J.P.; de Winter, D.; Bollati, V.; Milani, G.P. A safe flight for children through COVID-19 disaster: Keeping our mind open! Eur. J. Pediatr. 2020, 179, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Vanderbruggen, N.; Matthys, F.; Van Laere, S.; Zeeuws, D.; Santermans, L.; Van den Ameele, S.; Crunelle, C.L. Self-Reported Alcohol, Tobacco, and Cannabis Use during COVID-19 Lockdown Measures: Results from a Web-Based Survey. Eur. Addict. Res. 2020, 26, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Manavi, K.R.; Alston-Mills, B.P.; Thompson, M.P.; Allen, J.C. Effect of serum cotinine on vitamin D serum concentrations among american females with different ethnic backgrounds. Anticancer Res. 2015, 35, 1211–1218. [Google Scholar]
- Valtueña, J.; Gracia-Marco, L.; Vicente-Rodríguez, G.; González-Gross, M.; Huybrechts, I.; Rey-López, J.P.; Mouratidou, T.; Sioen, I.; Mesana, M.I.; Martínez, A.E.; et al. Vitamin D status and physical activity interact to improve bone mass in adolescents. The HELENA Study. Osteoporos. Int. 2012, 23, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Ferrari, A.; Targher, G. Is COVID-19 lockdown associated with vitamin D deficiency? Eur. J. Public Health 2021. [Google Scholar] [CrossRef]
- Brenner, H.; Holleczek, B.; Schöttker, B. Vitamin D Insufficiency and Deficiency and Mortality from Respiratory Diseases in a Cohort of Older Adults: Potential for Limiting the Death Toll during and beyond the COVID-19 Pandemic? Nutrients 2020, 12, 2488. [Google Scholar] [CrossRef]
- Rabufetti, A.; Milani, G.P.; Lava, S.A.G.; Edefonti, V.; Bianchetti, M.G.; Stettbacher, A.; Muggli, F.; Simonetti, G. Vitamin D Status Among Male Late Adolescents Living in Southern Switzerland: Role of Body Composition and Lifestyle. Nutrients 2019, 11, 2727. [Google Scholar] [CrossRef] [Green Version]
- Cuckson, A.C.; Reinders, A.; Shabeeh, H.; Shennan, A.H. Validation of the Microlife BP 3BTO-A oscillometric blood pressure monitoring device according to a modified British Hypertension Society protocol. Blood Press. Monit. 2002, 7, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Milani, G.P.; Simonetti, G.D.; Edefonti, V.; Lava, S.A.G.; Agostoni, C.; Curti, M.; Stettbacher, A.; Bianchetti, M.G.; Muggli, F. Seasonal variability of the vitamin D effect on physical fitness in adolescents. Sci. Rep. 2021, 11, 182. [Google Scholar] [CrossRef]
- Durazo-Arvizu, R.A.; Tian, L.; Brooks, S.P.J.; Sarafin, K.; Cashman, K.D.; Kiely, M.; Merkel, J.; Myers, G.L.; Coates, P.M.; Sempos, C.T. The Vitamin D Standardization Program (VDSP) Manual for Retrospective Laboratory Standardization of Serum 25-Hydroxyvitamin D Data. J. AOAC Int. 2017, 100, 1234–1243. [Google Scholar] [CrossRef]
- Burdette, C.Q.; Camara, J.E.; Nalin, F.; Pritchett, J.; Sander, L.C.; Carter, G.D.; Jones, J.; Betz, J.M.; Sempos, C.T.; Wise, S.A. Establishing an Accuracy Basis for the Vitamin D External Quality Assessment Scheme (DEQAS). J. AOAC Int. 2017, 100, 1277–1287. [Google Scholar] [CrossRef]
- Nagakumar, P.; Chadwick, C.L.; Bush, A.; Gupta, A. Collateral impact of COVID-19: Why should children continue to suffer? Eur. J. Pediatr. 2021. [Google Scholar] [CrossRef] [PubMed]
- DeLuccia, R.; Clegg, D.; Sukumar, D. The implications of vitamin D deficiency on COVID-19 for at-risk populations. Nutr. Rev. 2021, 79, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Santaolalla, A.; Beckmann, K.; Kibaru, J.; Josephs, D.; Van Hemelrijck, M.; Irshad, S. Association Between Vitamin D and Novel SARS-CoV-2 Respiratory Dysfunction—A Scoping Review of Current Evidence and Its Implication for COVID-19 Pandemic. Front. Physiol. 2020, 11, 564387. [Google Scholar] [CrossRef]
- Ferrante, G.; Camussi, E.; Piccinelli, C.; Senore, C.; Armaroli, P.; Ortale, A.; Garena, F.; Giordano, L. Did social isolation during the SARS-CoV-2 epidemic have an impact on the lifestyles of citizens? Epidemiol. Prev. 2020, 44, 353–362. [Google Scholar] [CrossRef]
- Nwosu, B.U.; Kum-Nji, P. Tobacco smoke exposure is an independent predictor of vitamin D deficiency in US children. PLoS ONE 2018, 13, e0205342. [Google Scholar] [CrossRef]
- González-Gross, M.; Valtueña, J.; Breidenassel, C.; Moreno, L.A.; Ferrari, M.; Kersting, M.; De Henauw, S.; Gottrand, F.; Azzini, E.; Widhalm, K.; et al. Vitamin D status among adolescents in Europe: The Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br. J. Nutr. 2012, 107, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Pollock, N.; Stallmann-Jorgensen, I.S.; Gutin, B.; Lan, L.; Chen, T.C.; Keeton, D.; Petty, K.; Holick, M.F.; Zhu, H. Low 25-hydroxyvitamin D levels in adolescents: Race, season, adiposity, physical activity, and fitness. Pediatrics 2010, 125, 1104–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Högström, M.; Nordström, A.; Nordström, P. Relationship between vitamin D metabolites and bone mineral density in young males: A cross-sectional and longitudinal study. Calcif. Tissue Int. 2006, 79, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.A. Assessing bone health in children and adolescents. Indian J. Endocrinol. Metab. 2012, 16, S205–S212. [Google Scholar] [CrossRef]
- Yılmaz, K.; Şen, V. Is vitamin D deficiency a risk factor for COVID-19 in children? Pediatr. Pulmonol. 2020, 55, 3595–3601. [Google Scholar] [CrossRef]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 2021. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.E.; Ginde, A.A. Vitamin D3 to Treat COVID-19: Different Disease, Same Answer. JAMA 2021. [Google Scholar] [CrossRef]
- Waldron, J.L.; Ashby, H.L.; Cornes, M.P.; Bechervaise, J.; Razavi, C.; Thomas, O.L.; Chugh, S.; Deshpande, S.; Ford, C.; Gama, R. Vitamin D: A negative acute phase reactant. J. Clin. Pathol. 2013, 66, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roso, M.B.; de Carvalho Padilha, P.; Mantilla-Escalante, D.C.; Ulloa, N.; Brun, P.; Acevedo-Correa, D.; Arantes Ferreira Peres, W.; Martorell, M.; Aires, M.T.; de Oliveira Cardoso, L.; et al. Covid-19 Confinement and Changes of Adolescent’s Dietary Trends in Italy, Spain, Chile, Colombia and Brazil. Nutrients 2020, 12, 1807. [Google Scholar] [CrossRef]
- Christensen, G.T.; Molbo, D.; Ängquist, L.H.; Mortensen, E.L.; Christensen, K.; Sørensen, T.I.; Osler, M. Cohort Profile: The Danish Conscription Database(DCD): A cohort of 728,160 men born from 1939 through 1959. Int. J. Epidemiol. 2015, 44, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Bursztyn, M. Isolated systolic hypertension in young adults: A heterogeneous finding. J. Hypertens. 2018, 36, 1791–1792. [Google Scholar] [CrossRef]

| 2014–2016 | 2020 | p-Value | |
|---|---|---|---|
| n | 437 | 298 | |
| Body weight, kg | 72.5 (65.7–84.0) | 72 (65.0–80.0) | 0.99 |
| Body height, m | 1.78 (1.73–1.82) | 1.77 (1.73–1.81) | 0.66 |
| Body mass index, kg/m2 | 23.0 (21.0–25.1) | 22.4 (20.9–25.3) | 0.50 |
| Waist circumference, cm | 81 (75–88) | 80 (75–87) | 0.06 |
| Blood pressure, mmHg | |||
| Systolic | 132 (123–136) | 125 (122–135) | 0.12 |
| Diastolic | 76 (70–82) | 73 (69–80) | 0.47 |
| Heart rate, beats/minute | 72 (66–84) | 71 (61–82) | 0.64 |
| Frequency of recreational physical activity | 0.02 | ||
| Never | 101 (23%) | 26 (8.7%) | |
| 1 per week | 75 (17%) | 86 (29%) | |
| 2–4 per week | 185 (42%) | 150 (50%) | |
| 5–6 per week | 41 (8.7%) | 27 (9.1%) | |
| Every day | 35 (10%) | 9 (3.0%) | |
| Smoking | 0.03 | ||
| Never | 249 (57) | 170 (57) | |
| 1–10 cigarettes/day | 126 (29) | 63 (21) | |
| 11–20 cigarettes/day | 67 (15) | 64 (21) | |
| >20 cigarettes/day | 5 (1.1) | 1 (0.3) | |
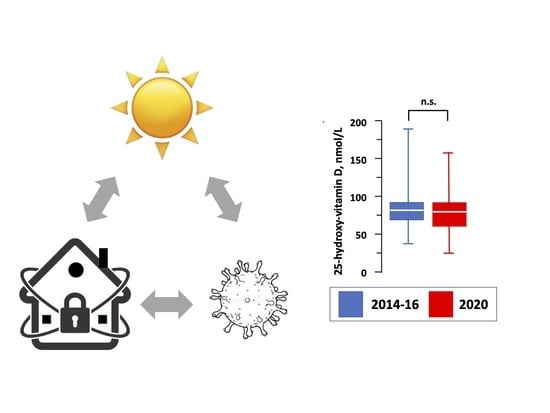
| Total 25-hydroxy-vitamin D, nmol/L | 77 (64–91) | 74 (60–92) | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meoli, M.; Muggli, F.; Lava, S.A.G.; Bianchetti, M.G.; Agostoni, C.; Kocher, C.; Bührer, T.W.; Ciliberti, L.; Simonetti, G.D.; Milani, G.P. Vitamin D Status in Adolescents during COVID-19 Pandemic: A Cross-Sectional Comparative Study. Nutrients 2021, 13, 1467. https://doi.org/10.3390/nu13051467
Meoli M, Muggli F, Lava SAG, Bianchetti MG, Agostoni C, Kocher C, Bührer TW, Ciliberti L, Simonetti GD, Milani GP. Vitamin D Status in Adolescents during COVID-19 Pandemic: A Cross-Sectional Comparative Study. Nutrients. 2021; 13(5):1467. https://doi.org/10.3390/nu13051467
Chicago/Turabian StyleMeoli, Martina, Franco Muggli, Sebastiano A.G. Lava, Mario G. Bianchetti, Carlo Agostoni, Claudine Kocher, Thomas W. Bührer, Letizia Ciliberti, Giacomo D. Simonetti, and Gregorio P. Milani. 2021. "Vitamin D Status in Adolescents during COVID-19 Pandemic: A Cross-Sectional Comparative Study" Nutrients 13, no. 5: 1467. https://doi.org/10.3390/nu13051467