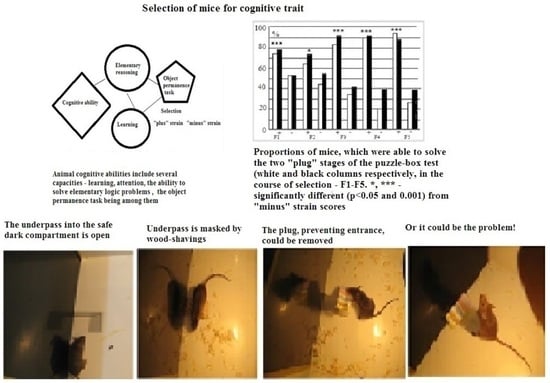
Selection of Mice for Object Permanence Cognitive Task Solution
Abstract
:1. Introduction
2. Material and Methods

 , 29
, 29 
 , F1 (“−”) 22
, F1 (“−”) 22 
 , 17
, 17 
 , F2 (“+”), 28
, F2 (“+”), 28 
 , 30
, 30 
 , F2 (“−”) 22
, F2 (“−”) 22 
 , 27
, 27 
 , F3 (“+”) 41
, F3 (“+”) 41 
 , 42
, 42 
 , F3 (“−”) 28
, F3 (“−”) 28 
 , 18
, 18 
 , F4 (“+”), 39
, F4 (“+”), 39 
 , 33
, 33 
 , F4 (“−”) 26
, F4 (“−”) 26 
 , 18
, 18 
 , F5 (“+”) 27
, F5 (“+”) 27 
 , 39
, 39 
 , F5 (“−”) 44
, F5 (“−”) 44 
 , 47
, 47 
 . Some animals in these animal groups from each generation were tested in the hyponeophagia test (the limited number of animals in this test was due to technical problems). Mice in the control, non-selected, heterogeneous populations from the respective generations were also tested in parallel with F4 and F5 of the selected strains (n = 97, 63
. Some animals in these animal groups from each generation were tested in the hyponeophagia test (the limited number of animals in this test was due to technical problems). Mice in the control, non-selected, heterogeneous populations from the respective generations were also tested in parallel with F4 and F5 of the selected strains (n = 97, 63 
 , 34
, 34 
 , in total), though F1–F3 control animals were not tested due to a technical problem.
, in total), though F1–F3 control animals were not tested due to a technical problem.3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crusio, W.E. Key issues in contemporary behavioral genetics. Curr. Opin. Behav. Sci. 2015, 2, 89–95. [Google Scholar] [CrossRef]
- Tryon, R.C. Genetic differences in maze learning ability in rats. Yearb. Nac. Soc. Stud. Educ. 1940, 39, 111–119. [Google Scholar]
- Shumskaia, I.A.; Marchenko, N.N.; Korochkin, L.I. The biochemical-genetic mechanisms of learning II. Selection for high and low rate of acquiring a motor conditioned reflex. Genetika 1975, 11, 74–80. [Google Scholar] [PubMed]
- Shumskaya, I.A.; Belyaev, A.I.; Korochkin, L.I. Analysis of hippocampal RNA in rats with genetically determined differences in learning ability. Neurosci Behav Physiol. 1981, 11, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Bignami, G. Selection for high rates and low rates of avoidance conditioning in the rat. Anim. Behav. 1965, 13, 221–227. [Google Scholar] [CrossRef]
- Brush, F.R. Selection for Differences in Avoidance Learning: The Syracuse Strains Differ in Anxiety, Not Learning Ability. Behav. Genet. 2003, 33, 677–696. [Google Scholar] [CrossRef]
- Steimer, T.; Driscoll, P. Inter-individual vs line/strain differences in psychogenetically selected Roman High-(RHA) and Low-(RLA) Avoidance rats: Neuroendocrine and behavioural aspects. Neurosci. Biobehav. Rev. 2005, 29, 99–112. [Google Scholar] [CrossRef]
- Ohta, R.; Kojima, K. Hatano rats selectively bred for high- and low-avoidance learning: An overview. Exp. Anim. 2019, 68, 127–136. [Google Scholar] [CrossRef]
- Bainbridge, N.K.; Koselke, L.R.; Jeon, J.; Bailey, K.R.; Wess, J.; Crawley, J.N.; Wrenn, C.C. Learning and memory impairments in a congenic C57BL/6 strain of mice that lacks the M2 muscarinic acetylcholine receptor subtype. Behav. Brain Res. 2008, 190, 50–58. [Google Scholar] [CrossRef]
- Graybeal, C.; Bachu, M.; Mozhui, K.; Saksida, L.M.; Bussey, T.J.; Sagalyn, E.; Williams, R.W.; Holmes, A. Strains and stressors: An analysis of touchscreen learning in genetically diverse mouse strains. PLoS ONE 2014, 9, e87745. [Google Scholar] [CrossRef]
- Saez, I.; Set, E.; Hsu, M. From genes to behavior: Placing cognitive models in the context of biological pathways. Front. Neurosci. 2014, 8, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lathe, R.; Morris, R.G.M. Analysing brain function and dysfunction in transgenic animals. Neuropathol. Appl. Neurobiol. 1994, 20, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Lipp, H.-P.; Wolfer, D.P. Genetically modified mice and cognition. Curr. Opin. Neurobiol. 1998, 8, 272–280. [Google Scholar] [CrossRef]
- Nguyen, P.V.; Abel, T.; Kandel, E.R.; Bourtchouladze, R. Strain-dependent Differences in LTP and Hippocampus-dependent Memory in Inbred Mice. Learn. Mem. 2000, 7, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Wolfer, D.P.; Lipp, H.-P. Dissecting the behavior of transgenic mice: Is it the mutation, the genetic background, or the environment? Exp. Physiol. 2000, 85, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Schimanski, L.A.; Nguyen, P.V. Multidisciplinary approaches for investigating the mechanisms of hippocampus-dependent memory: A focus on inbred mouse strains. Neurosci. Biobehav. Rev. 2004, 28, 463–483. [Google Scholar] [CrossRef]
- Wahlsten, D.; Cooper, S.F.; Crabbe, J.C. Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behav. Brain Res. 2005, 165, 36–51. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jang, Y.-S.; Jeon, W.K.; Han, J.-S. Assessment of Cognitive Phenotyping in Inbred, Genetically Modified Mice, and Transgenic Mouse Models of Alzheimer’s Disease. Exp. Neurobiol. 2019, 28, 146–157. [Google Scholar] [CrossRef]
- Poletaeva, I.I.; Popova, N.V.; Romanova, L.G. Genetic aspects of animal reasoning. Behav. Genet. 1993, 23, 467–475. [Google Scholar] [CrossRef]
- Galsworthy, M.J.; Paya-Cano, J.L.; Liu, L.; Monleón, S.; Gregoryan, G.; Fernandes, C.; Schalkwyk, L.; Plomin, R. Assessing Reliability, Heritability and General Cognitive Ability in a Battery of Cognitive Tasks for Laboratory Mice. Behav. Genet. 2005, 35, 675–692. [Google Scholar] [CrossRef]
- Ben Abdallah, N.M.; Fuss, J.; Trusel, M.; Galsworthy, M.G.; Bobsin, K.; Colacicco, G.; Deacon, R.M.J.; Riva, M.A.; Kellendonk, C.; Sprengel, R.; et al. The puzzle box as a simple and efficient behavioral test for exploring impairments of general cognition and executive functions in mouse models of schizophrenia. Exp. Neurol. 2011, 277, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Milos, N.; Vallortigara, G. Piagetian object permanence and its development in Eurasian jays (Garrulus glandarius). Anim. Cogn. 2007, 10, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Perepelkina, O.V.; Golibrodo, V.A.; Lilp, I.G.; Poletaeva, I.I. Selection of mice for high scores of elementary logical task solution. Dokl. Biol. Sci. 2015, 460, 52–56. [Google Scholar] [CrossRef]
- Perepelkina, O.V.; Poletaeva, I.I. Selection of Laboratory Mice for the Cognitive Task Successful Solution and for the Inability to Solve It. Dokl. Biochem. Biophys. 2021, 499, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Dulawa, S.C.; Hen, R. Recent advances in animal models of chronic antidepressant effects: The novelty-induced hypophagia test. Neurosci. Biobehav. Rev. 2005, 29, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Serra, A.; González-Soler, E.M.; Cervera-Ferri, A.; Teruel-Martí, V.; Valverde-Navarro, A.A. A standardization of the Novelty-Suppressed Feeding Test protocol in rats. Neurosci. Lett. 2017, 658, 73–78. [Google Scholar] [CrossRef]
- Ferre, P.; Fernández-Teruel, A.; Escorihuela, R.M.; Driscoll, P.; Corda, M.G.; Giorgi, O.; Tobeña, A. Behavior of the Roman/Verh high- and low-avoidance rat lines in anxiety tests: Relationship with defecation and self-grooming. Physiol. Behav. 1995, 58, 1209–1213. [Google Scholar] [CrossRef]
- Rutz, H.L.; Rothblat, L.A. Intact and impaired executive abilities in the BTBR mouse model of autism. Behav. Brain Res. 2012, 234, 33–37. [Google Scholar] [CrossRef]
- Chandler, D.J.; Waterhouse, B.D.; Gao, W.-J. New perspectives on catecholaminergic regulation of executive circuits: Evidence for independent modulation of prefrontal functions by midbrain dopaminergic and noradrenergic neurons. Front. Neur. Circ. 2014, 8, 53. [Google Scholar] [CrossRef]
- Royall, D.R.; Palmer, R.F. “Executive functions” cannot be distinguished from general intelligence: Two variations on a single theme within a symphony of latent variance. Front. Behav. Neurosci. 2014, 8, 369. [Google Scholar] [CrossRef]
- Yegla, B.; Foster, T.C.; Kumar, A. Behavior model for assessing decline in executive function during aging and neurodegenerative diseases. Methods Mol. Biol. 2019, 2011, 441–449. [Google Scholar] [PubMed]
- Lussier, A.A.; Bodnar, T.S.; Moksa, M.; Hirst, M.; Kobor, M.S.; Weinberg, J. Prenatal Adversity Alters the Epigenetic Profile of the Prefrontal Cortex: Sexually Dimorphic Effects of Prenatal Alcohol Exposure and Food-Related Stress. Genes 2021, 12, 1773. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.T.; Morris, R.G. Working memory(s). Brain Cogn. 1999, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.X. Genetic differences in leverpress escape/avoidance conditioning in seven mouse strains. Genes Brain Behav. 2004, 3, 110–114. [Google Scholar] [CrossRef]
- Matzel, L.D.; Kolata, S. Selective Attention, Working Memory, and Animal Intelligence. Neurosci. Biobehav. Rev. 2010, 34, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, P.A. An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 2004, 28, 699–709. [Google Scholar] [CrossRef]
- Matzel, L.D.; Han, Y.R.; Grossman, H.; Karnik, M.S.; Patel, D.; Scott, N.; Specht, S.M.; Gandhi, C.C. Individual differences in the expression of a “general” learning ability in mice. J. Neurosci. 2003, 23, 6423–6433. [Google Scholar] [CrossRef]
- Ponder, C.A.; Kliethermes, C.L.; Drew, M.R.; Muller, J.; Das, K.; Risbrough, V.B.; Crabbe, J.C.; Gilliam, T.C.; Palmer, A.A. Selection for contextual fear conditioning affects anxiety-like behaviors and gene expression. Genes Brain Behav. 2007, 6, 736–749. [Google Scholar] [CrossRef]
- Bushnell, P.J.; Levin, E.D.; Overstreet, D.H. Spatial working and reference memory in rats bred for autonomic sensitivity to cholinergic stimulation: Acquisition, accuracy, speed, and effects of cholinergic drugs. Neurobiol. Learn. Mem. 1995, 63, 116–132. [Google Scholar] [CrossRef]
- Lipp, H.-P.; Schwegler, H.; Heimrich, B.; Cerbone, A.; Sadile, A. Strain-specific correlations between hippocampal structural traits and habituation in a spatial novelty situation. Behav. Brain Res. 1987, 24, 111–123. [Google Scholar] [CrossRef]
- Lipp, H.-P.; Schwegler, H.; Crusio, W.E.; Wolfer, D.P.; Leisinger-Trigona, M.-C.; Heimrich, B.; Driscoll, P. Using genetically-defined rodent strains for the identification of hippocampal traits relevant for two-way avoidance behavior: A non-invasive approach. Experientia 1989, 45, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, D.; Reynolds, D.S.; Ferris, P.; Lincoln, R.; Datta, S.; Stanley, J.; Paterson, A.; Dawson, G.R.; Flint, J. Genetic Mapping of Variation in Spatial Learning in the Mouse. J. Neurosci. 2003, 23, 2426–2433. [Google Scholar] [CrossRef]
- Poletaeva, I.I.; Perepelkina, O.V.; Boyarshinova, O.S.; Golibrodo, V.A.; Lilp, I.G.; Lipp, H.-P.; Shin, H.S. The ability to solve elementary logic tasks in mice with the knockout of sodium–calcium exchanger gene 2 (NCX2). Dokl. Biol. Sci. 2016, 469, 159–162. [Google Scholar] [CrossRef]
- Paratore, S.E.; Alessi, C.S.; Torrisi, A.; Mastrobuono, F.; Cavallaro, S. Early genomics of learning and memory: A review. Genes Brain Behav. 2006, 5, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Thinschmidt, J.; Liu, J.; Ai, L.; Papke, R.L.; King, M.A.; Hughes, J.A.; Meyer, E.M. Alpha7 Nicotinic receptor gene delivery into mouse hippocampal neurons leads to functional receptor expression, improved spatial memory-related performance, and tau hyperphosphorylation. Neuroscience 2007, 145, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alcazar, M.; Daborg, J.; Stokowska, A.; Wasling, P.; Björefeldt, A.; Kalm, M.; Zetterberg, H.; Carlström, K.E.; Blomgren, K.; Ekdahl, C.T.; et al. Altered cognitive performance and synaptic function in the hippocampus of mice lacking C3. Exp. Neurol. 2014, 253, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.M.; Stead, J.D.H.; Miller, S.; Watson, S.J.; Akil, H. Developmental underpinnings of differences in rodent novelty seeking an emotional reactivity. Eur. J. Neurosci. 2011, 34, 994–1005. [Google Scholar] [CrossRef]
- Tully, T. Discovery of genes involved with learning and memory: An experimental synthesis of Hirschian and Benzerian perspectives. Proc. Natl. Acad. Sci. USA 1996, 93, 13460–13467. [Google Scholar] [CrossRef]
- Sauce, B.; Matzel, L.D. The paradox of intelligence: Heritability and malleability coexist in hidden gene–environment interplay. Psychol. Bull. 2018, 144, 26–47. [Google Scholar] [CrossRef]
- Anderson, B. The g factor in non-human animals. Novartis Found. Symp. 2000, 233, 79–90. [Google Scholar]
- Kang, J.; Shin, J.W.; Kim, Y.; Swanberg, K.M.; Kim, Y.; Bae, J.R.; Kim, Y.K.; Lee, J.; Kim, S.Y.; Sohn, N.W.; et al. Nobiletin improves emotional and novelty recognition memory but not spatial referential memory. J. Nat. Med. 2017, 71, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Caicoya, A.L.; Amici, F.; Ensenyat, C.; Colell, M. Comparative cognition in three understudied ungulate species: European bison, forest buffalos and giraffes. Front. Zool. 2021, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Langley, E.J.G.; Adams, G.; Beardsworth, C.E.; Dawson, D.A.; Laker, P.R.; van Horik, J.O.; Whiteside, M.A.; Wilson, A.J.; Madden, J.R. Heritability and correlations among learning and inhibitory control traits. Behav. Ecol. 2020, 31, 798–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perepelkina, O.V.; Poletaeva, I.I. Selection of Mice for Object Permanence Cognitive Task Solution. Neurol. Int. 2022, 14, 696-706. https://doi.org/10.3390/neurolint14030058
Perepelkina OV, Poletaeva II. Selection of Mice for Object Permanence Cognitive Task Solution. Neurology International. 2022; 14(3):696-706. https://doi.org/10.3390/neurolint14030058
Chicago/Turabian StylePerepelkina, Olga Viktorovna, and Inga Igorevna Poletaeva. 2022. "Selection of Mice for Object Permanence Cognitive Task Solution" Neurology International 14, no. 3: 696-706. https://doi.org/10.3390/neurolint14030058