Effective Oxygen Reduction Reaction Performance of FeCo Alloys In Situ Anchored on Nitrogen-Doped Carbon by the Microwave-Assistant Carbon Bath Method and Subsequent Plasma Etching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Samples
2.2. Characterizations
2.3. Electrochemical Measurements
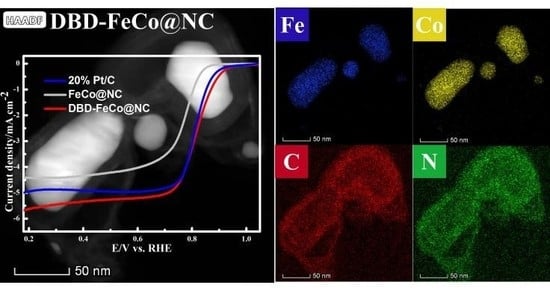
2.3.1. ORR Text
2.3.2. OER (Oxygen Evolution Reaction) Text
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Su, C.-Y.; Cheng, H.; Li, W.; Liu, Z.-Q.; Li, N.; Hou, Z.; Bai, F.-Q.; Zhang, H.-X.; Ma, T.-Y. Atomic Modulation of FeCo-Nitrogen-Carbon Bifunctional Oxygen Electrodes for Rechargeable and Flexible All-Solid-State Zinc-Air Battery. Adv. Energy Mater. 2017, 7, 1602420. [Google Scholar] [CrossRef]
- Zhang, X.; Lyu, D.; Mollamahale, Y.B.; Yu, F.; Qing, M.; Yin, S.; Zhang, X.; Tian, Z.Q.; Shen, P.K. Critical role of iron carbide nanodots on 3D graphene based nonprecious metal catalysts for enhancing oxygen reduction reaction. Electrochim. Acta 2018, 281, 502–509. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, Y.; Zhang, T.; Liu, Y.; Qiao, J. Efficient quantum dots anchored nanocomposite for highly active ORR/OER electrocatalyst of advanced metal-air batteries. Nano Energy 2019, 57, 176–185. [Google Scholar] [CrossRef]
- Zhao, Q.; Katyal, N.; Seymour, I.D.; Henkelman, G.; Ma, T. Vanadium(III) Acetylacetonate as an Efficient Soluble Catalyst for Lithium-Oxygen Batteries. Angew. Chem. Int. Ed. 2019, 58, 12553–12557. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Sun, X.; Xu, N.; Liu, J.; Wang, Y.; Qian, T.; Yan, C. Zinc-Air Batteries: Facilitated Oxygen Chemisorption in Heteroatom-Doped Carbon for Improved Oxygen Reaction Activity in All-Solid-State Zinc–Air Batteries (Adv. Mater. 4/2018). Adv. Mater. 2018, 30, 1870028. [Google Scholar] [CrossRef]
- Liu, M.; Guo, X.; Hu, L.; Yuan, H.; Wang, G.; Dai, B.; Zhang, L.; Yu, F. Fe3O4/Fe3C@nitrogen-doped carbon for enhancing oxygen reduction reaction. Chem. Nano Mat. 2019, 5, 187–193. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, C.; Liu, J.; Zeng, X.; Qu, S.; Han, X.; Deng, Y.; Hu, W.; Lu, J. Atomically Thin Mesoporous Co3 O4 Layers Strongly Coupled with N-rGO Nanosheets as High-Performance Bifunctional Catalysts for 1D Knittable Zinc-Air Batteries. Adv. Mat. 2017, 30, 1703657. [Google Scholar] [CrossRef]
- Hu, L.; Yu, F.; Yuan, H.; Wang, G.; Liu, M.; Wang, L.; Xue, X.; Peng, B.; Tian, Z.; Dai, B. Improved oxygen reduction reaction via a partially oxidized Co-CoO catalyst on N-doped carbon synthesized by a facile sand-bath method. Chin. Chem. Lett. 2019, 30, 624–629. [Google Scholar] [CrossRef]
- Han, J.; Meng, X.; Lu, L.; Bian, J.; Li, Z.; Sun, C. Single-Atom Fe-Nx-C as an Efficient Electrocatalyst for Zinc-Air Batteries. Adv. Funct. Mat. 2019. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Wang, G.; Dai, B.; Yu, F.; Tian, Z.; Guo, X. Enhanced Oxygen Reduction Reaction by In Situ Anchoring Fe2N Nanoparticles on Nitrogen-Doped Pomelo Peel-Derived Carbon. Nanomaterials 2017, 7, 404. [Google Scholar] [CrossRef]
- Shi, L.; Wu, T.; Wang, Y.; Zhang, J.; Wang, G.; Zhang, J.; Dai, B.; Yu, F. Nitrogen-Doped Carbon Nanoparticles for Oxygen Reduction Prepared via a Crushing Method Involving a High Shear Mixer. Materials 2017, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Du, X.X.; He, Y.; Wang, X.X.; Wang, J.N. Fine-grained and fully ordered intermetallic PtFe catalysts with largely enhanced catalytic activity and durability. Energy Environ. Sci. 2016, 9, 2623–2632. [Google Scholar] [CrossRef]
- Chong, L.; Wen, J.; Kubal, J.; Sen, F.G.; Zou, J.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W.; Liu, D.-J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 2018, 362, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhao, Z.; Cao, L.; Li, J.; Ghoshal, S.; Davies, V.; Stavitski, E.; Attenkofer, K.; Liu, Z.; Li, M.; et al. Roles of Mo Surface Dopants in Enhancing the ORR Performance of Octahedral PtNi Nanoparticles. Nano Lett. 2018, 18, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, Y.; Cao, Z.; Kuang, Q.; Du, G.; Jiang, Y.; Xie, Z.; Zheng, L. Excavated Cubic Platinum-Tin Alloy Nanocrystals Constructed from Ultrathin Nanosheets with Enhanced Electrocatalytic Activity. Angew. Chem. Int. Ed. 2016, 55, 9021–9025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-R.; Wöllner, S. Hollowed structured PtNi bifunctional electrocatalyst with record low total overpotential for oxygen reduction and oxygen evolution reactions. Appl. Catal. B Environ. 2018, 222, 26–34. [Google Scholar] [CrossRef]
- Wang, N.; Li, Y.; Guo, Z.; Li, H.; Hayase, S.; Ma, T. Minute quantities of hexagonal nanoplates PtFe alloy with facile operating conditions enhanced electrocatalytic activity and durability for oxygen reduction reaction. J. Alloy. Compd. 2018, 752, 23–31. [Google Scholar] [CrossRef]
- Zeng, M.; Liu, Y.; Zhao, F.; Nie, K.; Han, N.; Wang, X.; Huang, W.; Song, X.; Zhong, J.; Li, Y. Metallic Cobalt Nanoparticles Encapsulated in Nitrogen-Enriched Graphene Shells: Its Bifunctional Electrocatalysis and Application in Zinc-Air Batteries. Adv. Funct. Mater. 2016, 26, 4397–4404. [Google Scholar] [CrossRef]
- Bates, M.K.; Jia, Q.; Doan, H.; Liang, W.; Mukerjee, S. Charge-Transfer Effects in Ni–Fe and Ni–Fe–Co Mixed-Metal Oxides for the Alkaline Oxygen Evolution Reaction. ACS Catal. 2015, 6, 155–161. [Google Scholar] [CrossRef]
- Cai, P.; Ci, S.; Zhang, E.; Shao, P.; Cao, C.; Wen, Z. FeCo Alloy Nanoparticles Confined in Carbon Layers as High-activity and Robust Cathode Catalyst for Zn-Air Battery. Electrochim. Acta 2016, 220, 354–362. [Google Scholar] [CrossRef]
- Zhao, X.; Abbas, S.C.; Huang, Y.; Lv, J.; Wu, M.; Wang, Y. Robust and Highly Active FeNi@NCNT Nanowire Arrays as Integrated Air Electrode for Flexible Solid-State Rechargeable Zn-Air Batteries. Adv. Mater. Interfaces 2018, 5, 1701448. [Google Scholar] [CrossRef]
- Wei, L.; Karahan, H.E.; Zhai, S.; Liu, H.; Chen, X.; Zhou, Z.; Lei, Y.; Liu, Z.; Chen, Y. Amorphous Bimetallic Oxide-Graphene Hybrids as Bifunctional Oxygen Electrocatalysts for Rechargeable Zn-Air Batteries. Adv. Mater. 2017, 29, 1701410. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Lim, S.H.; Zhen, Y.; An, Y.; Lin, J. Optimized electrochemical performance of three-dimensional porous LiFePO 4 /C microspheres via microwave irradiation assisted synthesis. J. Power Sources 2014, 271, 223–230. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, L.; Lai, L.; Zhu, M.; Guo, Y.; Xia, L.; Qi, P.; Wang, G.; Dai, B. High Electrochemical Performance of LiFePO4 Cathode Material via In-Situ Microwave Exfoliated Graphene Oxide. Electrochim. Acta 2015, 151, 240–248. [Google Scholar] [CrossRef]
- Liu, M.; Yin, X.; Guo, X.; Hu, L.; Yuan, H.; Wang, G.; Wang, F.; Chen, L.; Zhang, L.; Yu, F. High efficient oxygen reduction performance of Fe/Fe3C nanoparticles in situ encapsulated in nitrogen-doped carbon via a novel microwave-assisted carbon bath method. Nano Mater. Sci. 2019, 1, 131–136. [Google Scholar] [CrossRef]
- Li, P.; Wen, B.; Yu, F.; Zhu, M.; Guo, X.; Han, Y.; Kang, L.; Huang, X.; Dan, J.; Ouyang, F.; et al. High efficient nickel/vermiculite catalyst prepared via microwave irradiation-assisted synthesis for carbon monoxide methanation. Fuel 2016, 171, 263–269. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, L.; Zhu, M.; An, Y.; Xia, L.; Wang, X.; Dai, B. Overwhelming microwave irradiation assisted synthesis of olivine-structured LiMPO4 (M=Fe, Mn, Co and Ni) for Li-ion batteries. Nano Energy 2014, 3, 64–79. [Google Scholar] [CrossRef]
- Ding, D.; Song, Z.-L.; Cheng, Z.-Q.; Liu, W.-N.; Nie, X.-K.; Bian, X.; Chen, Z.; Tan, W. Plasma-assisted nitrogen doping of graphene-encapsulated Pt nanocrystals as efficient fuel cell catalysts. J. Mater. Chem. A 2014, 2, 472–477. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, F.; Zhu, M.; Ma, C.; Zhao, D.; Wang, C.; Zhou, A.; Dai, B.; Ji, J.; Guo, X. N-Doping of plasma exfoliated graphene oxide via dielectric barrier discharge plasma treatment for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 2011–2017. [Google Scholar] [CrossRef]
- Lin, H.; Ruirui, Z.; Lingzhi, W.; Fapei, Z.; Qianwang, C. Synthesis of FeCo nanocrystals encapsulated in nitrogen-doped graphene layers for use as highly efficient catalysts for reduction reactions. Nanoscale 2014, 7, 450–454. [Google Scholar]
- Wang, X.; Wang, J.; Wang, D.; Dou, S.; Ma, Z.; Wu, J.; Tao, L.; Shen, A.; Ouyang, C.; Liu, Q.; et al. One-pot synthesis of nitrogen and sulfur co-doped graphene as efficient metal-free electrocatalysts for the oxygen reduction reaction. Chem. Commun. 2014, 50, 4839–4842. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Shen, A.; Tao, L.; Wang, S. Molecular doping of graphene as metal-free electrocatalyst for oxygen reduction reaction. Chem. Commun. 2014, 50, 10672–10675. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, G.; Chen, L.; Dai, B.; Yu, F. Three-Dimensional Honeycomb-Like Porous Carbon with Both Interconnected Hierarchical Porosity and Nitrogen Self-Doping from Cotton Seed Husk for Supercapacitor Electrode. Nanomaterials 2018, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Xu, X.X.; Hu, B.C.; Liang, H.W.; Lin, Y.; Chen, L.F.; Yu, S.H. Iron Carbide Nanoparticles Encapsulated in Mesoporous Fe-N-Doped Carbon Nanofibers for Efficient Electrocatalysis. Angew. Chem. Int. Ed. Engl. 2015, 54, 8179–8183. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Li, S.L.; Tang, Y.J.; Han, M.; Dai, Z.H.; Bao, J.C.; Lan, Y.Q. Nitrogen-doped Fe/Fe3C@graphitic layer/carbon nanotube hybrids derived from MOFs: efficient bifunctional electrocatalysts for ORR and OER. Chem. Commun. 2015, 51, 2710–2713. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.H.B.; Calegaro, M.L.; Ticianelli, E.A. Electrocatalytic activity of dispersed platinum and silver alloys and manganese oxides for the oxygen reduction in alkaline electrolyte. Russ. J. Electrochem. 2006, 42, 1283–1290. [Google Scholar] [CrossRef]
- Zeng, Y.; Lai, Z.; Han, Y.; Zhang, H.; Xie, S.; Lu, X. Oxygen-Vacancy and Surface Modulation of Ultrathin Nickel Cobaltite Nanosheets as a High-Energy Cathode for Advanced Zn-Ion Batteries. Adv. Mater. 2018, 1802396. [Google Scholar] [CrossRef]
- Lin, Q.; Bu, X.; Kong, A.; Mao, C.; Zhao, X.; Bu, F.; Feng, P. New Heterometallic Zirconium Metalloporphyrin Frameworks and Their Heteroatom-Activated High-Surface-Area Carbon Derivatives. J. Am. Chem. Soc. 2015, 137, 2235–2238. [Google Scholar] [CrossRef]
- Palaniselvam, T.; Kashyap, V.; Bhange, S.N.; Baek, J.-B.; Kurungot, S. Nanoporous Graphene Enriched with Fe/Co-N Active Sites as a Promising Oxygen Reduction Electrocatalyst for Anion Exchange Membrane Fuel Cells. Adv. Funct. Mater. 2016, 26, 2150–2162. [Google Scholar] [CrossRef]
- Liao, C.; Xu, Q.; Wu, C.; Fang, D.; Chen, S.; Chen, S.; Luo, J.; Li, L. Core–shell nano-structured carbon composites based on tannic acid for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 17215–17224. [Google Scholar] [CrossRef]
- Shen, A.; Zou, Y.; Wang, Q.; Dryfe, R.A.W.; Huang, X.; Dou, S.; Dai, L.; Wang, S. Oxygen Reduction Reaction in a Droplet on Graphite: Direct Evidence that the Edge Is More Active than the Basal Plane. Angew. Chem. Int. Ed. 2014, 53, 10804–10808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, P.; Tian, Z.; Zhu, M.; Wang, F.; Li, J.; Dai, B.; Yu, F.; Qiu, H.; Gao, H. Clarification of Active Sites at Interfaces between Silica Support and Nickel Active Components for Carbon Monoxide Methanation. Catalysts 2018, 8, 293. [Google Scholar] [CrossRef]
- Fu, H.; Liu, Y.; Chen, L.; Shi, Y.; Kong, W.; Hou, J.; Yu, F.; Wei, T.; Wang, H.; Guo, X. Designed formation of NiCo2O4 with different morphologies self-assembled from nanoparticles for asymmetric supercapacitors and electrocatalysts for oxygen evolution reaction. Electrochim. Acta 2019, 296, 719–729. [Google Scholar] [CrossRef]
- Xue, X.; Yu, F.; Peng, B.; Wang, G.; Lv, Y.; Chen, L.; Yao, Y.; Dai, B.; Shi, Y.; Guo, X. One-step synthesis of nickel–iron layered double hydroxides with tungstate acid anions via flash nano-precipitation for the oxygen evolution reaction. Sustain. Energy Fuels 2019, 3, 237–244. [Google Scholar] [CrossRef]
- Yan, J.; Kong, L.; Ji, Y.; White, J.; Li, Y.; Zhang, J.; An, P.; Liu, S.; Lee, S.-T.; Ma, T. Single atom tungsten doped ultrathin α-Ni(OH)2 for enhanced electrocatalytic water oxidation. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]





| Sample | Content (at.%) | Content of N Species (at.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | N | Fe | Co | Pyridinic | Pyrrolic | Graphitic | Oxidized | |
| FeCo@NC | 50.42 | 32.89 | 1.8 | 9.44 | 5.45 | 0.26 | 0.30 | 0.26 | 0.98 |
| DBD-FeCo@NC | 60.05 | 24.65 | 1.67 | 8.64 | 4.99 | 0.58 | 0.33 | 0.26 | 0.50 |
| Catalysts | Preparation Method | Onset Potential (V vs. RHE) | Half-Wave Potential (V vs. RHE) | Limiting-Current Density (mA/cm2) | Ref. |
|---|---|---|---|---|---|
| PtNi/C | Solution synthesis | - | 0.88 | - | [16] |
| PtFe alloy | Solution synthesis | 0.95 | 0.88 | 5.83 | [17] |
| FeCo@NC-750 | Furnace heating | 0.94 | 0.80 | 4.82 | [20] |
| FeNi@NCNTs | Furnace heating | 0.95 | 0.77 | 4.70 | [21] |
| FeCo@NC | MW-CBM | 0.88 | 0.78 | 4.42 | this work |
| DBD-FeCo@NC | MW-CBM | 0.96 | 0.88 | 5.66 | this work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Yu, F.; Ma, C.; Xue, X.; Fu, H.; Yuan, H.; Yang, S.; Wang, G.; Guo, X.; Zhang, L. Effective Oxygen Reduction Reaction Performance of FeCo Alloys In Situ Anchored on Nitrogen-Doped Carbon by the Microwave-Assistant Carbon Bath Method and Subsequent Plasma Etching. Nanomaterials 2019, 9, 1284. https://doi.org/10.3390/nano9091284
Liu M, Yu F, Ma C, Xue X, Fu H, Yuan H, Yang S, Wang G, Guo X, Zhang L. Effective Oxygen Reduction Reaction Performance of FeCo Alloys In Situ Anchored on Nitrogen-Doped Carbon by the Microwave-Assistant Carbon Bath Method and Subsequent Plasma Etching. Nanomaterials. 2019; 9(9):1284. https://doi.org/10.3390/nano9091284
Chicago/Turabian StyleLiu, Mincong, Feng Yu, Cunhua Ma, Xueyan Xue, Haihai Fu, Huifang Yuan, Shengchao Yang, Gang Wang, Xuhong Guo, and Lili Zhang. 2019. "Effective Oxygen Reduction Reaction Performance of FeCo Alloys In Situ Anchored on Nitrogen-Doped Carbon by the Microwave-Assistant Carbon Bath Method and Subsequent Plasma Etching" Nanomaterials 9, no. 9: 1284. https://doi.org/10.3390/nano9091284