In Vitro Modulation of Spontaneous Activity in Embryonic Cardiomyocytes Cultured on Poly(vinyl alcohol)/Bioglass Type 58S Electrospun Scaffolds
Abstract
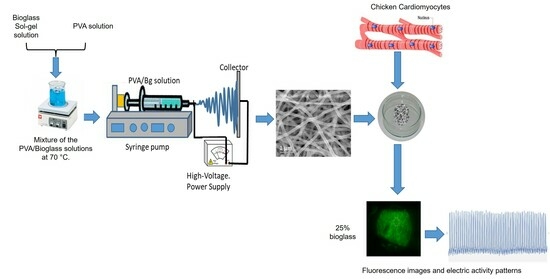
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. 58S Bioglass Sol-Gel Synthesis
2.1.2. Electrospinning of PVA and Hybrid Scaffolds
2.1.3. Chemical Crosslinking of PVA/Bg Scaffolds
2.2. Methods
2.2.1. Characterization of Non-Crosslinked and Crosslinked PVA/Bg Hybrid Scaffolds
2.2.2. Cardiomyocyte Culture on PVA/Bg Scaffolds
2.2.3. Fluorescence Analysis
3. Results and Discussion
3.1. Characterization of Electrospun PVA/Bg Hybrid Scaffolds
3.1.1. Nanofiber Diameter and Morphology by SEM
3.1.2. Elemental Mapping of Bg Particles by TEM
3.1.3. Functional Group Analysis by FTIR-ATR
3.1.4. Thermal Analysis by DSC and TGA
Differential Scanning Analysis, DSC
Thermogravimetric Analysis, TGA
3.1.5. Biological Interaction of Ca2+ in Hybrid Scaffolds with Cardiomyocytes
3.1.6. Proposed Model of Ca2+ Ion Dissolution with Time for the Interaction with Cardiomyocytes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kargozar, S.; Hamzehlou, S.; Baino, F. Potential of Bioactive Glasses for Cardiac and Pulmonary Tissue Engineering. Materials 2017, 10, 1429. [Google Scholar] [CrossRef]
- Deaths from Cardiovascular Diseases Surges 60% Globaly on the Last 30 Years: Report; World Heart Federation: Geneva, Switzerland, 2023.
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef]
- Freytes, D.O.; Santambrogio, L.; Vunjak-Novakovic, G. Optimizing Dynamic Interactions between a Cardiac Patch and Inflammatory Host Cells. Cells Tissues Organs 2011, 195, 171–182. [Google Scholar] [CrossRef]
- Maria, T.M.C.; de Carvalho, R.A.; Sobral, P.J.A.; Habitante, A.M.B.Q.; Solorza-Feria, J. The Effect of the Degree of Hydrolysis of the PVA and the Plasticizer Concentration on the Color, Opacity, and Thermal and Mechanical Properties of Films Based on PVA and Gelatin Blends. J. Food Eng. 2008, 87, 191–199. [Google Scholar] [CrossRef]
- Pogwizd, S.M.; Schlotthauer, K.; Li, L.; Yuan, W.; Bers, D.M. Arrhythmogenesis and Contractile Dysfunction in Heart Failure. Circ. Res. 2001, 88, 1159–1167. [Google Scholar] [CrossRef]
- Dattola, E.; Parrotta, E.I.; Scalise, S.; Perozziello, G.; Limongi, T.; Candeloro, P.; Coluccio, M.L.; Maletta, C.; Bruno, L.; De Angelis, M.T.; et al. Development of 3D PVA Scaffolds for Cardiac Tissue Engineering and Cell Screening Applications. RSC Adv. 2019, 9, 4246–4257. [Google Scholar] [CrossRef]
- Hirt, M.N.; Hansen, A.; Eschenhagen, T. Cardiac Tissue Engineering: State of the Art. Circ. Res. 2014, 114, 354–367. [Google Scholar] [CrossRef]
- Kitsara, M.; Agbulut, O.; Kontziampasis, D.; Chen, Y.; Menasché, P. Fibers for Hearts: A Critical Review on Electrospinning for Cardiac Tissue Engineering. Acta Biomater. 2017, 48, 20–40. [Google Scholar] [CrossRef]
- Cui, Z.; Yang, B.; Li, R.K. Application of Biomaterials in Cardiac Repair and Regeneration. Engineering 2016, 2, 141–148. [Google Scholar] [CrossRef]
- Ruvinov, E.; Cohen, S. Alginate Biomaterial for the Treatment of Myocardial Infarction: Progress, Translational Strategies, and Clinical Outlook. From Ocean Algae to Patient Bedside. Adv. Drug Deliv. Rev. 2016, 96, 54–76. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Wang, Y.; Lin, Q.; Yao, A.; Cao, F.; Li, D.; Zhou, J.; Duan, C.; Du, Z.; et al. The Influence of Chitosan Hydrogel on Stem Cell Engraftment, Survival and Homing in the Ischemic Myocardial Microenvironment. Biomaterials 2012, 33, 3093–3106. [Google Scholar] [CrossRef]
- Christman, K.L.; Fok, H.H.; Sievers, R.E.; Fang, Q.; Lee, R.J. Fibrin Glue Alone and Skeletal Myoblasts in a Fibrin Scaffold Preserve Cardiac Function after Myocardial Infarction. Tissue Eng. 2004, 10, 403–409. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Krishnan, U.M.; Sethuraman, S. Polymeric Scaffold Aided Stem Cell Therapeutics for Cardiac Muscle Repair and Regeneration. Macromol. Biosci. 2013, 13, 1119–1134. [Google Scholar] [CrossRef]
- Chiono, V.; Mozetic, P.; Boffito, M.; Sartori, S.; Gioffredi, E.; Silvestri, A.; Rainer, A.; Giannitelli, S.M.; Trombetta, M.; Nurzynska, D.; et al. Polyurethane-Based Scaffolds for Myocardial Tissue Engineering. Interface Focus 2014, 4, 20130045. [Google Scholar] [CrossRef]
- Plackett, D.; VÁzquez, A. Natural Polymer Sources. In Green Composites: Polymer Composites and the Environment; Woodhead Publishing: Sawston, UK, 2004; pp. 123–153. ISBN 9781855737396. [Google Scholar]
- Chaudhuri, R.; Ramachandran, M.; Moharil, P.; Harumalani, M.; Jaiswal, A.K. Biomaterials and Cells for Cardiac Tissue Engineering: Current Choices. Mater. Sci. Eng. C 2017, 79, 950–957. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.R.S. Crosslinked Poly(Vinyl Alcohol) Hydrogels for Wound Dressing Applications: A Review of Remarkably Blended Polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A Review of Polyvinyl Alcohol and Its Uses in Cartilage and Orthopedic Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100 B, 1451–1457. [Google Scholar] [CrossRef]
- Nagarkar, R.; Patel, J. Polyvinyl Alcohol: A Comprehensive Study. Acta Sci. Pharm. Sci. 2019, 3, 34–44. [Google Scholar]
- AM, A. A Mini-Review on the Bioactive Glass-Based Composites in Soft Tissue Repair. Bioceram. Dev. Appl. 2018, 8, 8–11. [Google Scholar] [CrossRef]
- Bui, X.V.; Dang, T.H. Bioactive Glass 58S Prepared Using an Innovation Sol-Gel Process. Process. Appl. Ceram. 2019, 13, 98–103. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Müller, T.; Schubert, D.W.; Boccaccini, A.R.; Yao, Q.; Roether, J.A. Electrospun Polyhydroxybutyrate/Poly(ε-Caprolactone)/58S Sol-Gel Bioactive Glass Hybrid Scaffolds with Highly Improved Osteogenic Potential for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17098–17108. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Calcium Fluxes Involved in Control of Cardiac Myocyte Contraction. Circ. Res. 2000, 87, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Ferguson, M.; Kamp, T.J.; Zhao, F. Constructing Biomimetic Cardiac Tissues: A Review of Scaffold Materials for Engineering Cardiac Patches. Emergent Mater. 2019, 2, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Biocompatibility Evaluation of Electrically Conductive Nanofibrous Scaffolds for Cardiac Tissue Engineering. J. Mater. Chem. B 2013, 1, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Liu, B.; Liu, J.; Liu, D.; Lu, Y.; Sun, X.; Liang, K.; Kong, B. Interfacial Tissue Engineering of Heart Regenerative Medicine Based on Soft Cell-Porous Scaffolds. J. Thorac. Dis. 2018, 10, S2333–S2345. [Google Scholar] [CrossRef] [PubMed]
- Fakoya, A.O.J. New Delivery Systems of Stem Cells for Vascular Regeneration in Ischemia. Front. Cardiovasc. Med. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Tallawi, M.; Rai, R.; Boccaccini, A.R.; Aifantis, K.E. Effect of Substrate Mechanics on Cardiomyocyte Maturation and Growth. Tissue Eng. Part B Rev. 2015, 21, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kong, C.W.; Tong, M.H.; Chooi, W.H.; Huang, N.; Li, R.A.; Chan, B.P. Maturation of Human Embryonic Stem Cell-Derived Cardiomyocytes (hESC-CMs) in 3D Collagen Matrix: Effects of Niche Cell Supplementation and Mechanical Stimulation. Acta Biomater. 2017, 49, 204–217. [Google Scholar] [CrossRef]
- Durán-Pastén, M.L.; González-Gómez, G.H. Recurrence Plots to Analyze the Dynamical Changes During. In AIP Conference Proceedings; AIP: Melville, NY, USA, 2019; Volume 2090, p. 050005. [Google Scholar]
- Li, Y.; Yao, S. High Stability under Extreme Condition of the Poly(Vinyl Alcohol) Nanofibers Crosslinked by Glutaraldehyde in Organic Medium. Polym. Degrad. Stab. 2017, 137, 229–237. [Google Scholar] [CrossRef]
- Durán-Pastén, M.L.; Cortes, D.; Valencia-Amaya, A.E.; King, S.; González-Gómez, G.H.; Hautefeuille, M. Cell Culture Platforms with Controllable Stiffness for Chick Embryonic Cardiomyocytes. Biomimetics 2019, 4, 33. [Google Scholar] [CrossRef]
- Oropeza-Ramos, L.; Macias, A.; Juárez, S.; Falcón, A.; Torres, A.; Hautefeuille, M.; González-Gómez, G.H. Low cost micro-platform for culturing and stimulation of cardiomyocyte tissue. In Proceedings of the 2011 IEEE 24th International Conference on Micro Electro Mechanical Systems, Cancun, Mexico, 23–27 January 2011; pp. 912–915. [Google Scholar]
- González, H.; Nagai, Y.; Bub, G.; Glass, L.; Shrier, A. Reentrant Waves in a Ring of Embryonic Chick Ventricular Cells Imaged with a Ca2+ Sensitive Dye. BioSystems 2003, 71, 71–80. [Google Scholar] [CrossRef]
- González, H.; Nagai, Y.; Bub, G.; Glass, L.; Shrier, A. Resetting and Annihilating Reentrant Waves in a Ring of Cardiac Tissue: Theory and Experiment. Prog. Theor. Phys. Suppl. 2000, 139, 83–89. [Google Scholar] [CrossRef]
- Bub, G.; Glass, L.; Publicover, N.G.; Shrier, A. Bursting Calcium Rotors in Cultured Cardiac Myocyte Monolayers. Proc. Natl. Acad. Sci. USA 1998, 95, 10283–10287. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Buendia, E.; González-Gómez, G.H.; Falcón-Neri, M.A.; Durán-Pastén, M.L.; Jiménez-Martínez, C.; Vera-Graziano, R.; Ospina-Orejarena, A.; Rivera-Torres, F.; Prado-Villegas, G.; Maciel-Cerda, A. Activity Patterns of Cardiomyocytes in Electrospun Scaffolds of Poly (ε-Caprolactone), Collagen, and Epicatechin. Mater. Today Commun. 2022, 31, 103405. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR Spectroscopy Characterization of Poly (Vinyl Alcohol) Hydrogel with Different Hydrolysis Degree and Chemically Crosslinked with Glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Tang, C.; Saquing, C.D.; Harding, J.R.; Khan, S.A. In Situ Cross-Linking of Electrospun Poly(Vinyl Alcohol) Nanofibers. Macromolecules 2010, 43, 630–637. [Google Scholar] [CrossRef]
- Figueiredo, K.C.S.; Alves, T.L.M.; Borges, C.P. Poly(Vinyl Alcohol) Films Crosslinked by Glutaraldehyde Under Mild Conditions. J. Appl. Polym. Sci. 2009, 111, 3074–3080. [Google Scholar] [CrossRef]
- Guirguis, O.W.; Moselhey, M.T.H. Thermal and Structural Studies of Poly (Vinyl Alcohol) and Hydroxypropyl Cellulose Blends. Nat. Sci. 2012, 4, 57–67. [Google Scholar] [CrossRef]
- Rault, J.; Gref, R.; Ping, Z.H.; Nguyen, Q.T.; Néel, J. Glass Transition Temperature Regulation Effect in a Poly(Vinyl Alcohol)-Water System. Polymer 1995, 36, 1655–1661. [Google Scholar] [CrossRef]
- Taghizadeh, M.T.; Yeganeh, N.; Rezaei, M. The Investigation of Thermal Decomposition Pathway and Products of Poly(Vinyl Alcohol) by TG-FTIR. J. Appl. Polym. Sci. 2015, 132, 42117. [Google Scholar] [CrossRef]
- Stavropoulou, A.; Papadokostaki, K.G.; Sanopoulou, M. Thermal Properties of Poly(Vinyl Alcohol)-Solute Blends Studied by TMDSC. J. Appl. Polym. Sci. 2004, 93, 1151–1156. [Google Scholar] [CrossRef]
- Huang, K.; Cai, S.; Xu, G.; Ren, M.; Wang, X.; Zhang, R.; Niu, S.; Zhao, H. Sol-Gel Derived Mesoporous 58S Bioactive Glass Coatings on AZ31 Magnesium Alloy and in Vitro Degradation Behavior. Surf. Coat. Technol. 2014, 240, 137–144. [Google Scholar] [CrossRef]
- Zanela, J.; Bilck, A.P.; Casagrande, M.; Grossmann, M.V.E.; Yamashita, F. Polyvinyl Alcohol (PVA) Molecular Weight and Extrusion Temperature in Starch/PVA Biodegradable Sheets. Polimeros 2018, 28, 256–265. [Google Scholar] [CrossRef]
- Peng, Z.; Kong, L.X. A Thermal Degradation Mechanism of Polyvinyl Alcohol/Silica Nanocomposites. Polym. Degrad. Stab. 2007, 92, 1061–1071. [Google Scholar] [CrossRef]
- Taghi Taghizadeh, M.; Sabouri, N. Thermal Degradation Behavior of Polyvinyl Alcohol/Starch/Carboxymethyl Cellulose/Clay Nanocomposites. Univers. J. Chem. 2013, 1, 21–29. [Google Scholar] [CrossRef]
- Aina, A.; Morris, A.; Gupta, M.; Billa, N.; Madhvani, N. Dissolution Behavior of Poly Vinyl Alcohol in Water and Its Effect on the Physical Morphologies of PLGA Scaffolds. Pharm. Biosci. J. 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Józó, M.; Simon, N.; Yi, L.; Móczó, J.; Pukánszky, B. Improved Release of a Drug with Poor Water Solubility by Using Electrospun Water-Soluble Polymers as Carriers. Pharmaceutics 2022, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Cerruti, M.G.; Greenspan, D.; Powers, K. An Analytical Model for the Dissolution of Different Particle Size Samples of Bioglass® in TRIS-Buffered Solution. Biomaterials 2005, 26, 4903–4911. [Google Scholar] [CrossRef] [PubMed]
- Miller-Chou, B.A.; Koenig, J.L. A Review of Polymer Dissolution. Prog. Polym. Sci. 2023, 28, 1223–1270. [Google Scholar] [CrossRef]
- Shapira, A.; Feiner, R.; Dvir, T. Composite Biomaterial Scaffolds for Cardiac Tissue Engineering. Int. Mater. Rev. 2016, 61, 1–19. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Guo, B.; Ma, P.X. Interwoven Aligned Conductive Nanofiber Yarn/Hydrogel Composite Scaffolds for Engineered 3D Cardiac Anisotropy. ACS Nano 2017, 11, 5646–5659. [Google Scholar] [CrossRef]
- Jin, G.; He, R.; Sha, B.; Li, W.; Qing, H.; Teng, R.; Xu, F. Electrospun Three-Dimensional Aligned Nanofibrous Scaffolds for Tissue Engineering. Mater. Sci. Eng. C 2018, 92, 995–1005. [Google Scholar] [CrossRef]
- Kim, H.N.; Hong, Y.; Kim, M.S.; Kim, S.M.; Suh, K.Y. Effect of Orientation and Density of Nanotopography in Dermal Wound Healing. Biomaterials 2012, 33, 8782–8792. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, X.; Snutch, T.P.; Tao, J. Modulation of Low-Voltage-Activated T-Type Ca2+ Channels. Biochim. Biophys. Acta Biomembr. 2013, 1828, 1550–1559. [Google Scholar] [CrossRef]
- Vassort, G.; Talavera, K.; Alvarez, J.L. Role of T-Type Ca2+ Channels in the Heart. Cell Calcium 2006, 40, 205–220. [Google Scholar] [CrossRef]
- Lieb, A.; Ortner, N.; Striessnig, J. C-Terminal Modulatory Domain Controls Coupling of Voltage-Sensing to Pore Opening in Cav1.3 L-Type Ca2+ Channels. Biophys. J. 2014, 106, 1467–1475. [Google Scholar] [CrossRef]
- Ono, K.; Iijima, T. Cardiac T-Type Ca2+ Channels in the Heart. J. Mol. Cell. Cardiol. 2010, 48, 65–70. [Google Scholar] [CrossRef]
- Putney, J.W. A Model for Receptor-Regulated Calcium Entry. Cell Calcium 1986, 7, 1–12. [Google Scholar] [CrossRef]
- Putney, J.W. Capacitative Calcium Entry Revisited. Review Article. Cell Calcium 1990, 11, 611–624. [Google Scholar] [CrossRef]
- Birkeland, J.A.; Sejersted, O.M.; Taraldsen, T.; Sjaastad, I. EC-Coupling in Normal and Failing Hearts. Scand. Cardiovasc. J. 2005, 39, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.; Bode, E.; Venetucci, L.; Trafford, A. Calcium Flux Balance in the Heart. J. Mol. Cell Cardiol. 2013, 58, 110–117. [Google Scholar] [CrossRef]
- Campbell, D.L.; Strauss, H.C. Regulation of Calcium Channels in the Heart. Adv. Second. Messenger Phosphoprot. Res. 1995, 30, 25–88. [Google Scholar] [CrossRef]










| Hybrid Scaffold Name | PVA/Bg Ratio (w/w) | CaO Concentration in Bg | Ca2+ Concentration in Bg |
|---|---|---|---|
| PVA | 100/0 | 0 | 0 |
| 5% | 95/5 | 0.1590 | 0.0114 |
| 10% | 90/10 | 0.3180 | 0.0227 |
| 15% | 85/15 | 0.0477 | 0.0341 |
| 20% | 80/20 | 0.0635 | 0.0454 |
| 25% | 75/25 | 0.0794 | 0.0567 |
| 30% | 70/30 | 0.0953 | 0.0681 |
| Bg Content in PVA, (%) | Non-Crosslinked PVA | Crosslinked PVA | |||
|---|---|---|---|---|---|
| TEv (°C) | Tg (°C) | Tm (°C) | Tg (°C) | Tm (°C) | |
| Pristine PVA | 50 | 63 | 218 | 66 | 218 |
| (a) 5% | - | 63 | 219 | 66 | 219 |
| (b) 10% | - | 70 | 205 | 68 | 262 |
| (c) 15% | - | 73 | 260 | 69 | 300 |
| (d) 20% | 60 | 78 | 263 | 78 | 300 |
| (e) 25% | 61 | 88 | 254 | 92 | 300 |
| (f) 30% | 56 | 86 | 261 | 98 | 300 |
| Bioglass | - | 385 | - | 385 | - |
| Bg Concentration in PVA, (%) | %Weight Loss before Crosslinking | %Weight Loss after Crosslinking | ||||
|---|---|---|---|---|---|---|
| Temperature Intervals (°C) | Temperature Intervals (°C) | |||||
| 40–200 | 200–350 | 350–600 | 40–200 | 200–350 | 350–600 | |
| 5 | 5 | 66 | 25 | 4.3 | 63.8 | 21.6 |
| 10 | 10 | 50 | 40 | 3 | 45 | 30 |
| 15 | 5 | 55 | 18 | 13 | 60 | 25 |
| 20 | 3 | 45 | 10 | 16 | 40 | 20 |
| 25 | 3 | 50 | 10 | 6 | 48 | 18 |
| 30 | 10 | 50 | 10 | 16 | 28 | 15 |
| PVA | 6 | 70 | 24 | 10 | 48 | 42 |
| Bg | 13.56 | 11 | - | 13.56 | 11 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Torres, F.; Maciel-Cerda, A.; González-Gómez, G.H.; Falcón-Neri, A.; Gómez-Lizárraga, K.; Esquivel-Posadas, H.T.; Vera-Graziano, R. In Vitro Modulation of Spontaneous Activity in Embryonic Cardiomyocytes Cultured on Poly(vinyl alcohol)/Bioglass Type 58S Electrospun Scaffolds. Nanomaterials 2024, 14, 372. https://doi.org/10.3390/nano14040372
Rivera-Torres F, Maciel-Cerda A, González-Gómez GH, Falcón-Neri A, Gómez-Lizárraga K, Esquivel-Posadas HT, Vera-Graziano R. In Vitro Modulation of Spontaneous Activity in Embryonic Cardiomyocytes Cultured on Poly(vinyl alcohol)/Bioglass Type 58S Electrospun Scaffolds. Nanomaterials. 2024; 14(4):372. https://doi.org/10.3390/nano14040372
Chicago/Turabian StyleRivera-Torres, Filiberto, Alfredo Maciel-Cerda, Gertrudis Hortensia González-Gómez, Alicia Falcón-Neri, Karla Gómez-Lizárraga, Héctor Tomás Esquivel-Posadas, and Ricardo Vera-Graziano. 2024. "In Vitro Modulation of Spontaneous Activity in Embryonic Cardiomyocytes Cultured on Poly(vinyl alcohol)/Bioglass Type 58S Electrospun Scaffolds" Nanomaterials 14, no. 4: 372. https://doi.org/10.3390/nano14040372