Water Adsorption Dynamics on Metal–Organic Framework MOF-801: Comparative Study of Loose and Glued Grains, and Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Synthesis
2.2. Adsorbent Characterization
2.3. Water Adsorption Dynamics
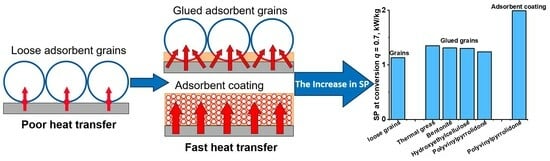
3. Results and Discussion
3.1. Characterization of the MOF-801 Consolidated Beds
3.2. Water Vapor Adsorption Dynamics
3.2.1. Glued MOF-801 Grains
3.2.2. MOF-801/Binder Coatings
3.3. Heat Transfer Coefficients
3.4. Specific Cooling Power
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- BP Statistical Review of World Energy 2022, 71st Edition. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2022-full-report.pdf (accessed on 17 August 2023).
- Wang, R.; Wang, L.; Wu, J. Adsorption Refrigeration Technology: Theory and Application; John Wiley & Sons, Singapore Pte. Ltd.: Singapore, 2014. [Google Scholar]
- Aristov, Y. Adsorptive transformation and storage of renewable heat: Review of current trends in adsorption dynamics. Renew. Energ. 2017, 110, 105–114. [Google Scholar] [CrossRef]
- He, F.; Nagano, K.; Seol, S.-H.; Togawa, J. Thermal performance improvement of AHP using corrugated heat exchanger by dip-coating method with mass recovery. Energy 2022, 239, 122418. [Google Scholar] [CrossRef]
- Calabrese, L.; Mittelbach, W.; Bonaccorsi, L.; Freni, A. An Industrial Approach for the Optimization of a New Performing Coated Adsorber for Adsorption Heat Pumps. Energies 2022, 15, 5118. [Google Scholar] [CrossRef]
- McCague, C.; Huttema, W.; Fradin, A.; Bahrami, M. Lab-scale sorption chiller comparison of FAM-Z02 coating and pellets. Appl. Therm. Eng. 2020, 173, 115219. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Aristov, Y.I. Adsorbent Coatings for Adsorption Heat Transformation: From Synthesis to Application. Energies 2022, 15, 7551. [Google Scholar] [CrossRef]
- Caprì, A.; Frazzica, A.; Calabrese, L. Recent Developments in Coating Technologies for Adsorption Heat Pumps: A Review. Coatings 2020, 10, 855. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Girnik, I.S.; Glaznev, I.S. Optimization of adsorption dynamics in adsorptive chillers: Loose grains configuration. Energy 2012, 46, 484–492. [Google Scholar] [CrossRef]
- Girnik, I.S.; Aristov, Y.I. Dynamic optimization of adsorptive chillers: The “AQSOA™-FAM-Z02-Water” working pair. Energy 2016, 106, 13–22. [Google Scholar] [CrossRef]
- Sharafian, A.; Fayazmanesh, K.; McCague, C.; Bahrami, M. Thermal conductivity and contact resistance of mesoporous silica gel adsorbents bound with polyvinylpyrrolidone in contact with a metallic substrate for adsorption cooling system applications. Int. J. Heat Mass Transf. 2014, 79, 64–71. [Google Scholar] [CrossRef]
- Guilleminot, J.J.; Choisier, A.; Chalfen, J.B.; Nicolast, S.; Reymoney, J.L. Heat transfer intensification in fixed bed adsorbers. Heat Recovery Syst. CHP 1993, 13, 297–300. [Google Scholar] [CrossRef]
- Kummer, H.; Baumgartner, M.; Hügenell, P.; Fröhlich, D.; Henninger, S.K.; Gläser, R. Thermally driven refrigeration by methanol adsorption on coatings of HKUST-1 and MIL-101(Cr). Appl. Therm. Eng. 2017, 117, 689–697. [Google Scholar] [CrossRef]
- Freni, A.; Bonaccorsi, L.; Calabrese, L.; Caprì, A.; Frazzica, A.; Sapienza, A. SAPO-34 coated adsorbent heat exchanger for adsorption chillers. Appl. Therm. Eng. 2015, 82, 1–7. [Google Scholar] [CrossRef]
- Li, A.; Thu, K.; Ismail, A.B.; Shahzad, M.V.; Ng, K.C. Performance of adsorbent-embedded heat exchangers using binder-coating method. Int. J. Heat Mass Transf. 2016, 92, 149–157. [Google Scholar] [CrossRef]
- Girnik, I.S.; Grekova, A.D.; Gordeeva, L.G.; Aristov, Y.I. Dynamic optimization of adsorptive chillers: Compact layer vs. bed of loose grains. Appl. Therm. Eng. 2017, 125, 823–829. [Google Scholar] [CrossRef]
- Frazzica, A.; Füldner, G.; Sapienza, A.; Freni, A.; Schnabel, L. Experimental and theoretical analysis of the kinetic performance of an adsorbent coating composition for use in adsorption chillers and heat pumps. Appl. Therm. Eng. 2014, 73, 1022–1031. [Google Scholar] [CrossRef]
- Girnik, I.S.; Aristov, Y.I. Dynamics of water vapour adsorption by a monolayer of loose AQSOA™-FAM-Z02 grains: Indication of inseparably coupled heat and mass transfer. Energy 2016, 114, 767–773. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Tu, Y.; Pan, Q.; Palash, M.L.; Saha, B.B.; Aristov, Y.I.; Wang, R. Metal-organic frameworks for energy conversion and water harvesting: A bridge between thermal engineering and material science. Nano Energy 2021, 84, 105946. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Kapteijn, F. Water and Metal–Organic Frameworks: From Interaction toward Utilization. Chem. Rev. 2020, 120, 8303–8377. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water adsorption in porous metal–organic frameworks and related materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef]
- Solovyeva, M.V.; Gordeeva, L.G.; Krieger, T.A.; Aristov, Y.I. MOF-801 as a promising material for adsorption cooling: Equilibrium and dynamics of water adsorption. Energy Conv. Manag. 2018, 174, 356–363. [Google Scholar] [CrossRef]
- Han, B.; Chakraborty, A. Tailoring Zirconium-based Metal Organic Frameworks for Enhancing Hydrophilic/Hydrophobic Characteristics: Simulation and Experimental Investigation. J. Mol. Liq. 2021, 341, 117381. [Google Scholar] [CrossRef]
- Jahan, I.; Islam, M.A.; Rupam, T.H.; Palash, M.L.; Rocky, K.A.; Saha, B.B. Enhanced water sorption onto bimetallic MOF-801 for energy conversion applications. SM&T 2022, 32, e00442. [Google Scholar]
- Jahan, I.; Rupam, T.H.; Palash, M.L.; Rocky, K.A.; Saha, B.B. Energy efficient green synthesized MOF-801 for adsorption cooling applications. J. Mol. Liq. 2022, 345, 117760. [Google Scholar] [CrossRef]
- Prasetya, N.; Li, K. Synthesis of defective MOF-801 via an environmentally benign approach for diclofenac removal from water streams. Sep. Purif. Technol. 2022, 301, 122024. [Google Scholar] [CrossRef]
- Aziz, A.N.; Al-Dadah, R.; Mahmoud, S.; Ismail, M.A.; Almesfer, M.K.; El-Kady, M.F.; Shokry, H. MOF-801/Graphene Adsorbent Material for Greenhouse Climate Control System—Numerical Investigation. Energies 2023, 16, 3864. [Google Scholar] [CrossRef]
- Almassad, H.A.; Abaza, R.I.; Siwwan, L.; Al-Maythalony, B.; Cordova, K.E. Environmentally adaptive MOF-based device enables continuous self-optimizing atmospheric water harvesting. Nat. Commun. 2022, 13, 4873. [Google Scholar] [CrossRef]
- An, L.; Liu, X.; Deng, B.; Jiang, H.; Cheng, G.J. Liquid metal nanolayer-linked MOF nanocomposites by laser shock evaporation. Matter 2021, 4, 3977–3990. [Google Scholar] [CrossRef]
- Taddei, M.; McPherson, M.J.; Gougsa, A.; Lam, J.; Sewell, J.; Andreoli, E. An Optimised Compaction Process for Zr-Fumarate (MOF-801). Inorganics 2019, 7, 110. [Google Scholar] [CrossRef]
- Gökpinar, S.; Ernst, S.-J.; Hastürk, E.; Möllers, M.; El Aita, I.; Wiedey, R.; Tannert, N.; Nießing, S.; Abdpour, S.; Schmitz, A.; et al. Air-con metal-organic frameworks in binder composites for water adsorption heat transformation systems. Ind. Eng. Chem. Res. 2019, 58, 21493–21503. [Google Scholar] [CrossRef]
- He, Y.; Fu, T.; Wang, L.; Liu, J.; Liu, G.; Zhao, H. Self-assembly of MOF-801 into robust hierarchically porous monoliths for scale-up atmospheric water harvesting. Chem. Eng. J. 2023, 472, 144786. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Dawoud, B.; Glaznev, I.S.; Elyas, A. A new methodology of studying the dynamics of water sorption/desorption under real operating conditions of adsorption heat pumps: Experiment. Int. J. Heat Mass Transf. 2008, 51, 4966–4972. [Google Scholar] [CrossRef]
- Working Report 2013-29: A Review of Porosity and Diffusion in Bentonite. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/45/087/45087776.pdf (accessed on 3 August 2023).
- Jovanovic, N.; Janackovic, J. Pore structure and adsorption properties of an acid-activated bentonite. Appl. Clay Sci. 1991, 6, 59–68. [Google Scholar] [CrossRef]
- Karger, J.; Ruthven, D.M.; Theodorou, D.N. Diffusion in Nanoporous Materials; Wiley-VCH Verlag: Weinheim, Germany, 2012. [Google Scholar]
- Knutsson, S. On the Thermal Conductivity and Thermal Diffusivity of Highly Compacted Bentonite; SKB Report-83-72; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 1983. [Google Scholar]
- Manufacturer’s Specification. Available online: https://nomacon.ru/katalog-tovarov/teploprovodyashhie-elektroizolyacionnye-materialy-kptd/kompaundy-zalivochnye-teploprovodyashhie-elektroizolyacionnye-nomakon-kptd-1.html (accessed on 17 August 2023).
- Xie, X.; Li, D.; Tsai, T.; Liu, J.; Braun, P.V.; Cahill, D.G. Thermal Conductivity, Heat Capacity, and Elastic Constants of Water- Soluble Polymers and Polymer Blends. Macromolecules 2016, 49, 972–978. [Google Scholar] [CrossRef]
- Wu, Y.; Ye, K.; Liu, Z.; Wang, M.; Chee, K.W.A.; Lin, C.; Jiang, N.; Yu, J. Effective thermal transport highway construction within dielectric polymer composites via a vacuum-assisted infiltration method. J. Mater. Chem. C 2018, 6, 6494–6501. [Google Scholar] [CrossRef]
- Strelova, S.; Gordeeva, L.; Aristov, Y. Dynamics of water vapour sorption on composite LiCl/(silica gel): An innovative configuration of the adsorbent bed. Energy, 2023; submitted. [Google Scholar]
- Grekova, A.D.; Veselovskaya, J.V.; Tokarev, M.M.; Gordeeva, L.G. Novel ammonia sorbents “porous matrix modified by active salt” for adsorptive heat transformation: 5. Designing the composite adsorbent for ice makers. Appl. Therm. Eng. 2012, 37, 80–86. [Google Scholar] [CrossRef]



 ), MOF-801_G/PVA_4.5 (
), MOF-801_G/PVA_4.5 ( ), MOF-801_G/PVP_4.5 (
), MOF-801_G/PVP_4.5 ( ), MOF-801_G/bent_4.5 (
), MOF-801_G/bent_4.5 ( ), MOF-801_G/HEC_4.5 (
), MOF-801_G/HEC_4.5 ( ), MOF-801_G/PAN_4.5 (
), MOF-801_G/PAN_4.5 ( ), and the reference bed of loose grains MOF-801_G_4.5 (
), and the reference bed of loose grains MOF-801_G_4.5 ( ). Dgr = 0.8–0.9 mm.
). Dgr = 0.8–0.9 mm.
 ), MOF-801_G/PVA_4.5 (
), MOF-801_G/PVA_4.5 ( ), MOF-801_G/PVP_4.5 (
), MOF-801_G/PVP_4.5 ( ), MOF-801_G/bent_4.5 (
), MOF-801_G/bent_4.5 ( ), MOF-801_G/HEC_4.5 (
), MOF-801_G/HEC_4.5 ( ), MOF-801_G/PAN_4.5 (
), MOF-801_G/PAN_4.5 ( ), and the reference bed of loose grains MOF-801_G_4.5 (
), and the reference bed of loose grains MOF-801_G_4.5 ( ). Dgr = 0.8–0.9 mm.
). Dgr = 0.8–0.9 mm.
 ), glued grains MOF-801_G/PVP(10)_4.5 (
), glued grains MOF-801_G/PVP(10)_4.5 ( ) and loose grains MOF-801-G_4.5 (
) and loose grains MOF-801-G_4.5 ( ).
).
 ), glued grains MOF-801_G/PVP(10)_4.5 (
), glued grains MOF-801_G/PVP(10)_4.5 ( ) and loose grains MOF-801-G_4.5 (
) and loose grains MOF-801-G_4.5 ( ).
).
 ), MOF-801_C/PVP(10)_8.9 (
), MOF-801_C/PVP(10)_8.9 ( ), MOF-801_C/bentonite(40)_8.9 (
), MOF-801_C/bentonite(40)_8.9 ( ), MOF-801_C/bentonite(20)_8.9 (
), MOF-801_C/bentonite(20)_8.9 ( ), and the reference bed of loose grains MOF-801-G_8.9 (
), and the reference bed of loose grains MOF-801-G_8.9 ( ).
).
 ), MOF-801_C/PVP(10)_8.9 (
), MOF-801_C/PVP(10)_8.9 ( ), MOF-801_C/bentonite(40)_8.9 (
), MOF-801_C/bentonite(40)_8.9 ( ), MOF-801_C/bentonite(20)_8.9 (
), MOF-801_C/bentonite(20)_8.9 ( ), and the reference bed of loose grains MOF-801-G_8.9 (
), and the reference bed of loose grains MOF-801-G_8.9 ( ).
).

| Sample | Cb [wt%] | Ssp * [m2/g] | Vp * [cm3/g] | Vμ * [cm3/g] | δ, % |
| MOF-801 as prepared | - | 900 | 0.49 | 0.27 | - |
| MOF-801_G | - | 820 | 0.45 | 0.23 | - |
| Glued grains, 0.8–0.9 mm | |||||
| MOF-801_G/PVA | 10 | 757 | 0.43 | 0.22 | 4 |
| MOF-801_G/PVP | 10 | 774 | 0.45 | 0.24 | 0 |
| MOF-801_G/PAN | 5 | 803 | 0.46 | 0.24 | 0 |
| MOF-801_G/AON | 40 | 724 | 0.41 | 0.21 | 9 |
| MOF-801_G/bent | 40 | 863 | 0.57 | 0.24 | −27 |
| MOF-801_G/CPTD | 40 | 380 | 0.32 | 0.11 | 29 |
| MOF-801_G/Aerocool | 40 | 732 | 0.42 | 0.27 | 7 |
| MOF-801_G/HEC | 10 | 695 | 0.40 | 0.19 | 11 |
| Coatings | |||||
| MOF-801_C/PVP(5) | 5 | 858 | 0.44 | 0.26 | 10 |
| MOF-801_C/PVP(10) | 10 | 830 | 0.43 | 0.25 | 12 |
| MOF-801_C/bent(20) | 20 | 954 | 0.54 | 0.28 | −10 |
| MOF-801_C/bent(40) | 40 | 1089 | 0.67 | 0.37 | −37 |
| Configuration | τ1, s | τ2, s | A | t0.7, s | t0.8, s | Δwt→∝, * g/g |
|---|---|---|---|---|---|---|
| Adsorption | ||||||
| MOF-801_G_4.5 | 88 | 170 | 0.21 | 170 | 245 | 0.21 |
| MOF-801_G/CPTD_4.5 | 87 | 210 | 0.29 | 185 | 270 | 0.20 |
| MOF-801_G/Aerocool_4.5 | 67 | 160 | 0.23 | 140 | 210 | 0.20 |
| MOF-801_G/bent_4.5 | 81 | 150 | 0.15 | 155 | 220 | 0.22 |
| MOF-801_G/PVA_4.5 | 130 | 330 | 0.21 | 320 | 460 | 0.20 |
| MOF-801_G/PVP_4.5 | 69 | 160 | 0.29 | 140 | 200 | 0.21 |
| MOF-801_G/PAN_4.5 | 113 | 200 | 0.15 | 220 | 300 | 0.20 |
| MOF-801_G/HEC_4.5 | 77 | 130 | 0.20 | 130 | 185 | 0.20 |
| Desorption | ||||||
| MOF-801-G_4.5 | 117 | 480 | 0.68 | 150 | 240 | 0.23 |
| MOF-801_G/CPTD_4.5 | 98 | 290 | 0.61 | 130 | 200 | 0.23 |
| MOF-801_G/Aerocool_4.5 | 91 | 310 | 0.64 | 115 | 185 | 0.22 |
| MOF-801_G/bent_4.5 | 103 | 300 | 0.63 | 130 | 195 | 0.24 |
| MOF-801_G/PVA_4.5 | 127 | 320 | 0.69 | 150 | 215 | 0.22 |
| MOF-801_G/PVP_4.5 | 108 | 460 | 0.70 | 145 | 235 | 0.23 |
| MOF-801_G/PAN_4.5 | 145 | 270 | 0.53 | 175 | 250 | 0.24 |
| MOF-801_G/HEC_4.5 | 106 | 360 | 0.67 | 135 | 205 | 0.22 |
| Sample | τ1, s | τ2, s | A | t0.7, s | t0.8, s | Δwt→∝, * g/g |
|---|---|---|---|---|---|---|
| Adsorption | ||||||
| MOF-801-G_4.5 | 88 | 173 | 0.21 | 170 | 245 | 0.21 |
| MOF-801_C/PVP(10)_ 4.5 | 87 | 241 | 0.79 | 115 | 148 | 0.20 |
| MOF-801-G_8.9 | 50 | 155 | 0.61 | 72 | 111 | 0.18 |
| MOF-801_C/PVP(10)_8.9 | 28 | 68 | 0.60 | 40 | 59 | 0.20 |
| MOF-801_C/PVP(5)_8.9 | 38 | 49 | 0.18 | 48 | 67 | 0.22 |
| MOF-801_C/bent(40)_8.9 | 58 | 94 | 0.18 | 94 | 134 | 0.22 |
| MOF-801_C/bent(20)_8.9 | 59 | 84 | 0.15 | 98 | 135 | 0.22 |
| Desorption | ||||||
| MOF-801-G_4.5 | 103 | 476 | 0.68 | 150 | 240 | 0.23 |
| MOF-801_C/PVP(10)_4.5 | 50 | 540 | 0.82 | 61 | 88 | 0.21 |
| MOF-801-G_8.9 | 33 | 507 | 0.78 | 47 | 69 | 0.18 |
| MOF-801_C/PVP(10)_8.9 | 20 | 602 | 0.77 | 32 | 56 | 0.21 |
| MOF-801_C/PVP(5)_8.9 | 15 | 637 | 0.80 | 21 | 31 | 0.23 |
| MOF-801_C/bent(40)_8.9 | 31 | 633 | 0.76 | 64 | 122 | 0.24 |
| MOF-801_C/bent(20)_8.9 | 27 | 787 | 0.77 | 49 | 94 | 0.24 |
| Configuration | Wmax, kW/kg | SCP0.8, kW/kg | SCP0.7, kW/kg | |
|---|---|---|---|---|
| Adsorption | Desorption | |||
| Loose grains | ||||
| MOF-801_G_4.5 | 7.8 | 7.4 | 0.85 | 1.13 |
| MOF-801_G_8.9 | 12.0 | 18.3 | 1.95 | 2.60 |
| Glued grains | ||||
| MOF-801_G/CPTD_4.5 | 7.6 | 7.7 | 0.84 | 1.10 |
| MOF-801_G/Aerocool_4.5 | 10.0 | 8.1 | 1.01 | 1.35 |
| MOF-801_G/PVA_4.5 | 5.0 | 5.8 | 0.58 | 0.72 |
| MOF-801_G/bentonite_4.5 | 8.3 | 7.7 | 1.06 | 1.31 |
| MOF-801_G/HEC_4.5 | 8.6 | 6.9 | 1.01 | 1.30 |
| MOF-801_G/PAN_4.5 | 5.9 | 5.5 | 0.73 | 0.90 |
| MOF-801_G/PVP_4.5 | 10.0 | 7.1 | 0.95 | 1.24 |
| Coatings | ||||
| MOF-801_C/PVP(10)_4.5 | 7.9 | 13.8 | 1.66 | 1.99 |
| MOF-801_C/PVP(10)_8.9 | 23.6 | 35.4 | 3.26 | 4.60 |
| MOF-801_C/PVP(5)_8.9 | 18.8 | 44.6 | 4.09 | 5.16 |
| MOF-801_C/bent(40)_8.9 | 12.7 | 25.7 | 1.64 | 2.32 |
| MOF-801_C/bent(20)_8.9 | 12.3 | 29.2 | 1.86 | 2.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solovyeva, M.; Gordeeva, L. Water Adsorption Dynamics on Metal–Organic Framework MOF-801: Comparative Study of Loose and Glued Grains, and Coatings. Nanomaterials 2023, 13, 2442. https://doi.org/10.3390/nano13172442
Solovyeva M, Gordeeva L. Water Adsorption Dynamics on Metal–Organic Framework MOF-801: Comparative Study of Loose and Glued Grains, and Coatings. Nanomaterials. 2023; 13(17):2442. https://doi.org/10.3390/nano13172442
Chicago/Turabian StyleSolovyeva, Marina, and Larisa Gordeeva. 2023. "Water Adsorption Dynamics on Metal–Organic Framework MOF-801: Comparative Study of Loose and Glued Grains, and Coatings" Nanomaterials 13, no. 17: 2442. https://doi.org/10.3390/nano13172442