Effect of Nanoparticle Size and Concentration on Pool Boiling Heat Transfer with TiO2 Nanofluids on Laser-Textured Copper Surfaces
Abstract
:1. Introduction
1.1. Methods for Intensifying Boiling Heat Transfer
1.2. Boiling of Nanofluids
1.3. Boiling on Laser-Texured Surfaces
1.4. Scope and Aim of this Study
2. Methods
2.1. Sample Preparation and Amalysis
2.2. Boiling Performance Evaluation
2.3. Data Reduction and Measurement Uncertainty
2.4. Peparation of Nanofluids
2.5. Measurement Protocol
3. Results and Discussion
3.1. Boiling Heat Transfer with Water
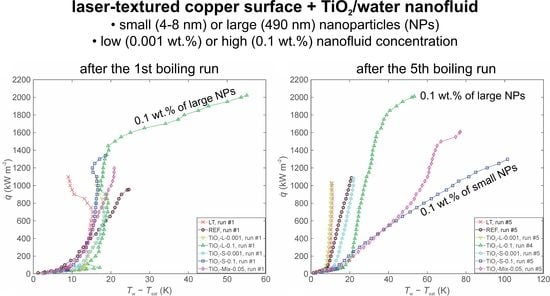
3.2. The Effect of Concentration on Boiling of Nanofluid with Small and Large TiO2 Nanoparticles
3.3. Contact Angle Measurments
3.4. Comparison of the Overall Boiling Performance
4. Conclusions
- The boiling of nanofluids on an already enhanced (i.e., laser-textured) surface failed to provide a notable (additional) enhancement of the heat transfer coefficient.
- At a low nanoparticle concentration, the influence of nanofluid on boiling performance is minimal, with heat transfer coefficient and CHF values comparable to those obtained using pure water on both the untreated and laser-textured surface.
- The boiling of a nanofluid with a high nanoparticle concentration resulted in significant deposition of nanoparticles onto the boiling surface and CHF enhancement up to 2021 kW m−2, representing double the value obtained on the untreated reference surface using water. However, very high surface superheat values (up to 100 K) were recorded, suggesting poor practical applicability.
- The decrease in heat transfer performance due to the boiling of nanofluids on laser-textured surfaces can be explained through the deposition of nanoparticles into the laser-induced grooves and microcavities present on the surface, which decreased the number of active nucleation sites. Furthermore, thicker nanoparticle deposits resulted in added thermal resistance. While the surface porosity granted a notable delay in CHF incipience due to enhanced liquid replenishment, the surface superheat was massively increased.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dhir, V.K. Nucleate and Transition Boiling Heat Transfer Under Pool and External Flow Conditions. Int. J. Heat Fluid Flow 1991, 12, 129–155. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of spray cooling—Part 1: Single-phase and nucleate boiling regimes, and critical heat flux. Int. J. Heat Mass Transf. 2017, 115, 1174–1205. [Google Scholar] [CrossRef]
- Mori, S.; Utaka, Y. Critical heat flux enhancement by surface modification in a saturated pool boiling: A review. Int. J. Heat Mass Transf. 2017, 108, 2534–2557. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, F.; Li, X.; Chen, Y. Gravity–capillary evaporation regimes in microgrooves. AIChE J. 2019, 65, 1119–1125. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Wu, R.; Shi, M. Flow boiling in constructal tree-shaped minichannel network. Int. J. Heat Mass Transf. 2011, 54, 202–209. [Google Scholar] [CrossRef]
- Mudawar, I. Recent advances in high-flux, two-phase thermal management. J. Therm. Sci. Eng. Appl. 2013, 5, 021012. [Google Scholar] [CrossRef]
- Nukiyama, S. The maximum and minimum values of the heat Q transmitted from metal to boiling water under atmospheric pressure. Int. J. Heat Mass Transf. 1966, 9, 1419–1433. [Google Scholar] [CrossRef]
- Lahey, R.T., Jr. Boiling Heat Transfer: Modern Developments and Advances, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Hewitt, G.F.; Tien, C.L. Series in Chemical and Mechanical Engineering Tong and Tang, Boiling Heat Transfer and Two-Phase Flow, 2nd ed.; Routledge: New York, NY, USA, 1997. [Google Scholar]
- Malavasi, I.; Teodori, E.; Moita, A.S.; Moreira, A.L.N.; Marengo, M. Wettability Effect on Pool Boiling: A Review. In Encyclopedia of Two-Phase Heat Transfer and Flow III; World Scientific Publishing Co. Pte Ltd.: Singapore, 2018; pp. 1–61. [Google Scholar]
- Liang, G.; Mudawar, I. Review of pool boiling enhancement by surface modification. Int. J. Heat Mass Transf. 2019, 128, 892–933. [Google Scholar] [CrossRef]
- Pioro, I.L.; Rohsenow, W.; Doerffer, S.S. Nucleate pool-boiling heat transfer. I: Review of parametric effects of boiling surface. Int. J. Heat Mass Transf. 2004, 47, 5033–5044. [Google Scholar] [CrossRef]
- Može, M.; Vajc, V.; Zupančič, M.; Golobič, I. Hydrophilic and hydrophobic nanostructured copper surfaces for efficient pool boiling heat transfer with water, water/butanol mixtures and Novec 649. Nanomaterials 2021, 11, 3216. [Google Scholar] [CrossRef]
- Zupančič, M.; Može, M.; Gregorčič, P.; Golobič, I. Nanosecond laser texturing of uniformly and non-uniformly wettable micro structured metal surfaces for enhanced boiling heat transfer. Appl. Surf. Sci. 2017, 399, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Chu, K.H.; Enright, R.; Wang, E.N. Structured surfaces for enhanced pool boiling heat transfer. Appl. Phys. Lett. 2012, 100, 241603. [Google Scholar] [CrossRef]
- Ji, X.; Xu, J.; Zhao, Z.; Yang, W. Pool boiling heat transfer on uniform and non-uniform porous coating surfaces. Exp. Therm. Fluid Sci. 2013, 48, 198–212. [Google Scholar] [CrossRef]
- Li, J.Q.; Mou, L.W.; Zhang, J.Y.; Zhang, Y.H.; Fan, L.W. Enhanced pool boiling heat transfer during quenching of water on superhydrophilic porous surfaces: Effects of the surface wickability. Int. J. Heat Mass Transf. 2018, 125, 494–505. [Google Scholar] [CrossRef]
- Lee, C.Y.; Zhang, B.J.; Kim, K.J. Morphological change of plain and nano-porous surfaces during boiling and its effect on nucleate pool boiling heat transfer. Exp. Therm. Fluid Sci. 2012, 40, 150–158. [Google Scholar] [CrossRef]
- Shi, J.; Jia, X.; Feng, D.; Chen, Z.; Dang, C. Wettability effect on pool boiling heat transfer using a multiscale copper foam surface. Int. J. Heat Mass Transf. 2020, 146, 118726. [Google Scholar] [CrossRef]
- Li, S.; Furberg, R.; Toprak, M.S.; Palm, B.; Muhammed, M. Nature-inspired boiling enhancement by novel nanostructured macroporous surfaces. Adv. Funct. Mater. 2008, 18, 2215–2220. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, S.D.; Bang, I.C. Critical heat flux for CuO nanofluid fabricated by pulsed laser ablation differentiating deposition characteristics. Int. J. Heat Mass Transf. 2012, 55, 6908–6915. [Google Scholar] [CrossRef]
- Ahn, H.S.; Sathyamurthi, V.; Banerjee, D. Pool boiling experiments on a nano-structured surface. IEEE Trans. Compon. Packag. Technol. 2009, 32, 156–165. [Google Scholar] [CrossRef]
- Gupta, S.K.; Misra, R.D. Experimental study of pool boiling heat transfer on copper surfaces with Cu-Al2O3 nanocomposite coatings. Int. Commun. Heat Mass Transf. 2018, 97, 47–55. [Google Scholar] [CrossRef]
- O’Connor, J.P.; You, S.M.; Chang, J.Y. Gas-Saturated Pool Boiling Heat Transfer from Smooth and Microporous Surfaces in FC-72. ASME J. Heat Transf. 1996, 118, 662–667. [Google Scholar] [CrossRef]
- Takata, Y.; Hidaka, S.; Masuda, M.; Ito, T. Pool boiling on a superhydrophilic surface. Int. J. Energy Res. 2003, 27, 111–119. [Google Scholar] [CrossRef]
- O’Hanley, H.; Coyle, C.; Buongiorno, J.; McKrell, T.; Hu, L.W.; Rubner, M.; Cohen, R. Separate effects of surface roughness, wettability, and porosity on the boiling critical heat flux. Appl. Phys. Lett. 2013, 103, 024102. [Google Scholar] [CrossRef]
- Kim, S.J.; Bang, I.C.; Buongiorno, J.; Hu, L.W. Effects of nanoparticle deposition on surface wettability influencing boiling heat transfer in nanofluids. Appl. Phys. Lett. 2006, 89, 153107. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.; Ahn, H.S.; Kang, S.; Kim, M.H. A study of nucleate boiling heat transfer on hydrophilic, hydrophobic and heterogeneous wetting surfaces. Int. J. Heat Mass Transf. 2011, 54, 5643–5652. [Google Scholar] [CrossRef]
- Phan, H.T.; Caney, N.; Marty, P.; Colasson, S.; Gavillet, J. Surface wettability control by nanocoating: The effects on pool boiling heat transfer and nucleation mechanism. Int. J. Heat Mass Transf. 2009, 52, 5459–5471. [Google Scholar] [CrossRef]
- Barber, J.; Brutin, D.; Tadrist, L. A review on boiling heat transfer enhancement with nanofluids. Nanoscale Res. Lett. 2011, 6, 280. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Chen, Y.; Zhang, H.; Chen, W.; Dong, A.; Wang, R. Heat transfer and critical heat flux of nanofluid boiling: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 62, 924–940. [Google Scholar] [CrossRef]
- Gerardi, C.; Buongiorno, J.; Hu, L.-W.; McKrell, T. Infrared thermometry study of nanofluid pool boiling phenomena. Nanoscale Res. Lett. 2011, 6, 232. [Google Scholar] [CrossRef] [Green Version]
- Vafaei, S.; Borca-Tasciuc, T. Role of nanoparticles on nanofluid boiling phenomenon: Nanoparticle deposition. Chem. Eng. Res. Des. 2014, 92, 842–856. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Nieto De Castro, C.A.; Loureno, M.J.V.; Lopes, M.L.M.; Santos, F.J.V. A review of boiling and convective heat transfer with nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 2342–2354. [Google Scholar] [CrossRef]
- Khan, A.; Ali, H.M. A comprehensive review pool boiling using nanofluids. Therm. Sci. 2019, 23, 3209–3237. [Google Scholar] [CrossRef]
- Dadhich, M.; Prajapati, O.S. A brief review on factors affecting flow and pool boiling. Renew. Sustain. Energy Rev. 2019, 112, 607–625. [Google Scholar] [CrossRef]
- Manetti, L.L.; Stephen, M.T.; Beck, P.A.; Cardoso, E.M. Evaluation of the heat transfer enhancement during pool boiling using low concentrations of Al2O3-water based nanofluid. Exp. Therm. Fluid Sci. 2017, 87, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, O.; Hamed, M.S. Experimental investigation of the effect of particle deposition on pool boiling of nanofluids. Int. J. Heat Mass Transf. 2012, 55, 3423–3436. [Google Scholar] [CrossRef]
- Huang, C.K.; Lee, C.W.; Wang, C.K. Boiling enhancement by TiO2 nanoparticle deposition. Int. J. Heat Mass Transf. 2011, 54, 4895–4903. [Google Scholar] [CrossRef]
- Kiyomura, I.S.; Manetti, L.L.; da Cunha, A.P.; Ribatski, G.; Cardoso, E.M. An analysis of the effects of nanoparticles deposition on characteristics of the heating surface and ON pool boiling of water. Int. J. Heat Mass Transf. 2017, 106, 666–674. [Google Scholar] [CrossRef] [Green Version]
- Salimpour, M.R.; Abdollahi, A.; Afrand, M. An experimental study on deposited surfaces due to nanofluid pool boiling: Comparison between rough and smooth surfaces. Exp. Therm. Fluid Sci. 2017, 88, 288–300. [Google Scholar] [CrossRef]
- Taylor, R.A.; Phelan, P.E. Pool boiling of nanofluids: Comprehensive review of existing data and limited new data. Int. J. Heat Mass Transf. 2009, 52, 5339–5347. [Google Scholar] [CrossRef]
- Park, S.D.; Moon, S.B.; Bang, I.C. Effects of thickness of boiling-induced nanoparticle deposition on the saturation of critical heat flux enhancement. Int. J. Heat Mass Transf. 2014, 78, 506–514. [Google Scholar] [CrossRef]
- Yim, K.; Lee, J.; Naccarato, B.; Kim, K.J. Surface wettability effect on nucleate pool boiling heat transfer with titanium oxide (TiO2) coated heating surface. Int. J. Heat Mass Transf. 2019, 133, 352–358. [Google Scholar] [CrossRef]
- Može, M.; Senegačnik, M.; Gregorčič, P.; Hočevar, M.; Zupančič, M.; Golobič, I. Laser-Engineered Microcavity Surfaces with a Nanoscale Superhydrophobic Coating for Extreme Boiling Performance. ACS Appl. Mater. Interfaces 2020, 12, 24419–24431. [Google Scholar] [CrossRef]
- Kruse, C.M.; Anderson, T.; Wilson, C.; Zuhlke, C.; Alexander, D.; Gogos, G.; Ndao, S. Enhanced pool-boiling heat transfer and critical heat flux on femtosecond laser processed stainless steel surfaces. Int. J. Heat Mass Transf. 2015, 82, 109–116. [Google Scholar] [CrossRef]
- Zakšek, P.; Zupančič, M.; Gregorčič, P.; Golobič, I. Investigation of Nucleate Pool Boiling of Saturated Pure Liquids and Ethanol-Water Mixtures on Smooth and Laser-Textured Surfaces. Nanoscale Microscale Thermophys. Eng. 2020, 24, 29–42. [Google Scholar] [CrossRef]
- Serdyukov, V.; Starinskiy, S.; Malakhov, I.; Safonov, A.; Surtaev, A. Laser texturing of silicon surface to enhance nucleate pool boiling heat transfer. Appl. Therm. Eng. 2021, 194, 117102. [Google Scholar] [CrossRef]
- Može, M.; Zupančič, M.; Hočevar, M.; Golobič, I.; Gregorčič, P. Surface chemistry and morphology transition induced by critical heat flux incipience on laser-textured copper surfaces. Appl. Surf. Sci. 2019, 490, 220–230. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Coulombe, S.; Kietzig, A.M. Boiling heat transfer enhancement with stable nanofluids and laser textured copper surfaces. Int. J. Heat Mass Transf. 2018, 126, 287–296. [Google Scholar] [CrossRef]
- Freitas, E.; Pontes, P.; Cautela, R.; Bahadur, V.; Miranda, J.; Ribeiro, A.P.C.; Souza, R.R.; Oliveira, J.D.; Copetti, J.B.; Lima, R.; et al. Article pool boiling of nanofluids on biphilic surfaces: An experimental and numerical study. Nanomaterials 2021, 11, 125. [Google Scholar] [CrossRef]
- Pontes, P.; Freitas, E.; Fernandes, D.; Teixeira, A.; Ferreira, R.; Bellmann, S.; Cautela, R.; Moita, A.S.; Bahadur, V.; Miranda, J.; et al. Pool boiling of nanofluids on biphilic surfaces. In Proceedings of the 8th European Thermal Sciences Conference (EUROTHERMAL 2021), Virtual, 20–22 September 2021; Journal of Physics: Conference Series. 2021; Volume 2116. [Google Scholar] [CrossRef]
- Može, M.; Vajc, V.; Zupančič, M.; Šulc, R.; Golobič, I. Pool boiling performance of water and self-rewetting fluids on hybrid functionalized aluminum surfaces. Processes 2021, 9, 1058. [Google Scholar] [CrossRef]
- Može, M.; Zupančič, M.; Golobič, I. Investigation of the scatter in reported pool boiling CHF measurements including analysis of heat flux and measurement uncertainty evaluation methodology. Appl. Therm. Eng. 2020, 169, 114938. [Google Scholar] [CrossRef]
- Može, M.; Zupančič, M.; Golobič, I. Pattern geometry optimization on superbiphilic aluminum surfaces for enhanced pool boiling heat transfer. Int. J. Heat Mass Transf. 2020, 161, 120265. [Google Scholar] [CrossRef]
- Gregorčič, P.; Conradi, M.; Hribar, L.; Hočevar, M. Long-term influence of laser-processing parameters on (Super)hydrophobicity development and stability of stainless-steel surfaces. Materials 2018, 11, 2240. [Google Scholar] [CrossRef] [Green Version]
- Trdan, U.; Hočevar, M.; Gregorčič, P. Transition from superhydrophilic to superhydrophobic state of laser textured stainless steel surface and its effect on corrosion resistance. Corros. Sci. 2017, 123, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Ta, D.V.; Dunn, A.; Wasley, T.J.; Kay, R.W.; Stringer, J.; Smith, P.J.; Connaughton, C.; Shephard, J.D. Nanosecond laser textured superhydrophobic metallic surfaces and their chemical sensing applications. Appl. Surf. Sci. 2015, 357, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Zhong, M.; Fan, P.; Gong, D.; Zhang, H. Wettability conversion of ultrafast laser structured copper surface. J. Laser Appl. 2015, 27, S29107. [Google Scholar] [CrossRef] [Green Version]
- Gregorčič, P. Comment on “Bioinspired Reversible Switch between Underwater Superoleophobicity/Superaerophobicity and Oleophilicity/Aerophilicity and Improved Antireflective Property on the Nanosecond Laser-Ablated Superhydrophobic Titanium Surfaces”. ACS Appl. Mater. Interfaces 2020, 13, 2117–2127. [Google Scholar] [CrossRef] [Green Version]
- Vig, J.R.; Bus, J.W.L. UV/Ozone Cleaning of Surfaces. IEEE Trans. Parts Hybrids Packag. 1976, 12, 365–370. [Google Scholar] [CrossRef]
- Vafaei, S. Nanofluid pool boiling heat transfer phenomenon. Powder Technol. 2015, 277, 181–192. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Hormozi, F.; Peyghambarzadeh, S.M. Pool boiling heat transfer to aqueous alumina nano-fluids on the plain and concentric circular micro-structured (CCM) surfaces. Exp. Therm. Fluid Sci. 2016, 72, 125–139. [Google Scholar] [CrossRef]
- Coursey, J.S.; Kim, J. Nanofluid boiling: The effect of surface wettability. Int. J. Heat Fluid Flow 2008, 29, 1577–1585. [Google Scholar] [CrossRef]
- Park, K.J.; Jung, D.; Shim, S.E. Nucleate boiling heat transfer in aqueous solutions with carbon nanotubes up to critical heat fluxes. Int. J. Multiph. Flow 2009, 35, 525–532. [Google Scholar] [CrossRef]
- Kathiravan, R.; Kumar, R.; Gupta, A.; Chandra, R. Characterization and pool boiling heat transfer studies of nanofluids. J. Heat Transf. 2009, 131, 081902. [Google Scholar] [CrossRef]
- Ciloglu, D.; Bolukbasi, A. A comprehensive review on pool boiling of nanofluids. Appl. Therm. Eng. 2015, 84, 45–63. [Google Scholar] [CrossRef]
- Kim, H.D.; Kim, M.H. CHF Enhancement in Pool Boiling of Nanofluid: Effect of Nanoparticle-Coating on Heating Surface. In Proceedings of the KNS spring meeting, Jeju, Korea, 26–27 May 2005. [Google Scholar]
- Suriyawong, A.; Wongwises, S. Nucleate pool boiling heat transfer characteristics of TiO2-water nanofluids at very low concentrations. Exp. Therm. Fluid Sci. 2010, 34, 992–999. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; He, Y.; Liu, Z.; Zhao, Y. Effect of nanoparticle size and concentration on boiling performance of SiO2 nanofluid. Int. J. Heat Mass Transf. 2017, 107, 820–828. [Google Scholar] [CrossRef]
- Soltani, S.; Etemad, S.G.; Thibault, J. Pool boiling heat transfer performance of Newtonian nanofluids. Heat Mass Transf. Stoffuebertragung 2009, 45, 1555–1560. [Google Scholar] [CrossRef]
- Das, S.K.; Prakash Narayan, G.; Baby, A.K. Survey on nucleate pool boiling of nanofluids: The effect of particle size relative to roughness. J. Nanoparticle Res. 2008, 10, 1099–1108. [Google Scholar] [CrossRef]
















| Name | Boiling Fluid | Surface | Size of Nanoparticles | Concentration (wt.%) |
|---|---|---|---|---|
| REF | water | Bare copper | / | / |
| LT | water | laser textured copper | / | / |
| TiO2-S-0.001 | TiO2-water | small (4–8 nm) | 0.001 | |
| TiO2-S-0.1 | TiO2-water | large (490 nm) | 0.1 | |
| TiO2-L-0.001 | TiO2-water | small (4–8 nm) | 0.001 | |
| TiO2-L-0.1 | TiO2-water | large (490 nm) | 0.1 | |
| TiO2-Mix-0.05 | TiO2-water | small (4–8 nm) + large (490 nm) | 0.05 small and 0.05 large |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadžić, A.; Može, M.; Arhar, K.; Zupančič, M.; Golobič, I. Effect of Nanoparticle Size and Concentration on Pool Boiling Heat Transfer with TiO2 Nanofluids on Laser-Textured Copper Surfaces. Nanomaterials 2022, 12, 2611. https://doi.org/10.3390/nano12152611
Hadžić A, Može M, Arhar K, Zupančič M, Golobič I. Effect of Nanoparticle Size and Concentration on Pool Boiling Heat Transfer with TiO2 Nanofluids on Laser-Textured Copper Surfaces. Nanomaterials. 2022; 12(15):2611. https://doi.org/10.3390/nano12152611
Chicago/Turabian StyleHadžić, Armin, Matic Može, Klara Arhar, Matevž Zupančič, and Iztok Golobič. 2022. "Effect of Nanoparticle Size and Concentration on Pool Boiling Heat Transfer with TiO2 Nanofluids on Laser-Textured Copper Surfaces" Nanomaterials 12, no. 15: 2611. https://doi.org/10.3390/nano12152611