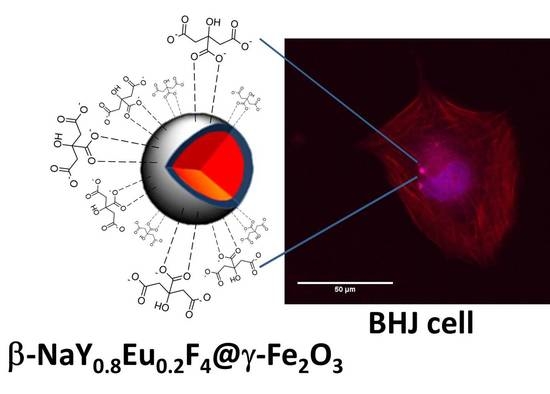
Polyol-Made Luminescent and Superparamagnetic β-NaY0.8Eu0.2F4@γ-Fe2O3 Core-Satellites Nanoparticles for Dual Magnetic Resonance and Optical Imaging
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Water-Soluble β-NaY0.8Eu0.2F4@γ-Fe2O3 Nanoprobes
2.2. Structural and Microstructural Characterization
2.3. Magnetometry and Relaxometry Measurements
2.4. Photoluminescence Measurements
2.5. Cell Culture and in Cellulo Cytotoxicity Assay
2.6. Confocal and Two-Photon Microscopies
3. Results and Discussion
3.1. Structural and Microstructural Properties
3.2. Magnetic Properties
3.3. Optical Properties
3.4. In Cellulo Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.-J. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Investig. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wáng, Y.X.J.; Idée, J.-M. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, P.C.; Daniel-da-Silva, A.L.; Tavares, D.S.; Calatayud, M.P.; Goya, G.F.; Trindade, T. Fluorescent Magnetic Bioprobes by Surface Modification of Magnetite Nanoparticles. Materials 2013, 6, 3213–3225. [Google Scholar] [CrossRef] [PubMed]
- Piraux, H.; Hai, J.; Verbeke, P.; Serradji, N.; Ammar, S.; Losno, R.; Ha-Duong, N.-T.; Hémadi, M.; Chahine, J.-M.E. Nanoparticles and the Transferrin-Receptor-1 iron-Acquisition Pathway—Synthesis, Functionalization, Kinetics, Thermodynamics and Cellular Internalization of a Holotraferrin-maghemite construction. Biochim. Biophys. Acta 2013, 1830, 4254–4264. [Google Scholar] [CrossRef] [PubMed]
- Belkahla, H.; Haque, A.; Revzin, A.; Gharbi, T.; Constantinescu, A.; Micheau, O.; Hémadi, M.; Ammar, S. Coupling TRAIL to iron oxide nanoparticles increases its apoptotic activity on HCT116 and HepG2 cells: Effect of magnetic core size. J. Interdisciplinary Nanomed. 2019, 4, 35–50. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, H.; Lipowska, M.; Wang, L.; Yu, Q.; Yang, X.; Tiwari, D.; Yang, L.; Mao, H. A dual-modal magnetic nanoparticle probe for preoperative and intraoperative mapping of sentinel lymph nodes by magnetic resonance and near infrared fluorescence imaging. J. Biomater. Appl. 2013, 28, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.-H.; Yang, B.Y.; Kim, Y.J.; Hong, M.K.; Lee, Y.-S.; Lee, N.S.; Chung, J.-K.; Jeong, J.M. Development of a complementary PET/MR dual-modal imaging probe for targeting prostate-specific membrane antigen (PSMA). Nanomed. Nanotechnol. Boil. Med. 2016, 12, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Mulder, W.J.M.; Koole, R.; Brandwijk, R.J.; Storm, G.; Chin, P.T.K.; Strijkers, G.J.; Donega, C.D.M.; Nicolay, K.; Griffioen, A.W. Quantum Dots with a Paramagnetic Coating as a Bimodal Molecular Imaging Probe. Nano Lett. 2006, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ni, D.; Zhang, J.; Bu, W.; Xing, H.; Han, F.; Xiao, Q.; Yao, Z.; Chen, F.; He, Q.; Liu, J.; et al. Dual-Targeting Upconversion Nanoprobes across the Blood–Brain Barrier for Magnetic Resonance/Fluorescence Imaging of Intracranial Glioblastoma. ACS Nano 2014, 8, 1231–1242. [Google Scholar] [CrossRef]
- Rosenthal, E.L.; Warram, J.M.; Bland, K.I.; Zinn, K.R. The Status of Contemporary Image-Guided Modalities in Oncologic Surgery. Ann. Surg. 2015, 261, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.; Zhao, Y.; Wang, S.; Liu, M.; Duan, Z.; Chen, Y.; Li, F.; Xu, F.; Lu, T. Recent advances in synthesis and surface modification of lanthanide-doped upconversion nanoparticles for biomedical applications. Biotechnol. Adv. 2012, 30, 1551–1561. [Google Scholar] [CrossRef]
- Liang, S.; Liu, Y.; Tang, Y.; Xie, Y.; Sun, H.; Zhang, H.; Yang, B. A User-Friendly Method for Synthesizing High-Quality:Yb,Er(Tm) Nanocrystals in Liquid Paraffin. J. Nanomaterials 2011, 302364. [Google Scholar] [CrossRef] [Green Version]
- Sudheendra, L.; Ortalan, V.; Dey, S.; Browning, N.D.; Kennedy, I.M. Plasmonic Enhanced Emissions from Cubic NaYF4:Yb:Er/Tm Nanophosphors. Chem. Mater. 2011, 23, 2987–2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Li, M.; Xie, M.; Chen, L.; Li, Y.; Wang, Q. Dopant-controlled synthesis of water-soluble hexagonal NaYF4 nanorods with efficient upconversion fluorescence for multicolor bioimaging. Nano Res. 2010, 3, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Carron, S.; Li, Q.Y.; Elst, L.V.; Muller, R.N.; Parac-Vogt, T.N.; Capobianco, J.A. Assembly of near infra-red emitting upconverting nanoparticles and multiple Gd(iii)-chelates as a potential bimodal contrast agent for MRI and optical imaging. Dalton Trans. 2015, 44, 11331–11339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, R.; Peng, H.; Li, P.; Xu, Z.; Whittaker, A.K. The evolution of gadolinium based contrast agents: From single-modality to multi-modality. Nanoscale 2016, 8, 10491–10510. [Google Scholar] [CrossRef]
- Zheng, K.; Loh, K.Y.; Wang, Y.; Chen, Q.; Fan, J.; Jung, T.; Nam, S.H.; Suh, Y.D.; Liu, X. Recent advances in upconversion nanocrystals: Expanding the kaleidoscopic toolbox for emerging applications. Nano Today 2019, 29, 100797. [Google Scholar] [CrossRef]
- Xia, A.; Chen, M.; Gao, Y.; Wu, D.M.; Feng, W.; Li, F.Y. Gd3+ complex-modified NaLuF4-based upconversion nanophosphors for trimodality imaging of NIR-to-NIR upconversion luminescence, X-Ray computed tomography and magnetic resonance. Biomaterials 2012, 33, 5394–5405. [Google Scholar] [CrossRef]
- Baziulyte-Paulaviciene, D.; Karabanovas, V.; Stasys, M.; Jarockyte, G.; Poderys, V.; Sakirzanovas, S.; Rotomskis, R. Synthesis and functionalization of NaGdF4:Yb,Er@NaGdF4 core–shell nanoparticles for possible application as multimodal contrast agents. Beilstein J. Nanotechnol. 2017, 8, 1815–1824. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Wang, X.; Meng, L.; Bu, Y.; Yan, X. Enhance the Er3+ Upconversion Luminescence by Constructing NaGdF4:Er3+@NaGdF4:Er3+ Active-Core/Active-Shell Nanocrystals. Nanoscale Res. Lett. 2017, 12, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Zhu, X.; Peng, J.; Li, F. Core–Shell Lanthanide Upconversion Nanophosphors as Four-Modal Probes for Tumor Angiogenesis Imaging. ACS Nano 2013, 7, 11290–11300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; He, Y.; Liu, J.; Feng, J.; Sun, Z.; Lei, P.; Yuan, Q.; Zhang, H. Core–shell BaYbF5:Tm@BaGdF5:Yb,Tm nanocrystals for in vivo trimodal UCL/CT/MR imaging. RSC Adv. 2016, 6, 14283–14289. [Google Scholar] [CrossRef]
- Lu, H.; Yi, G.; Zhao, S.; Chen, D.; Guo, L.-H.; Cheng, J. Synthesis and characterization of multi-functional nanoparticles possessing magnetic, up-conversion fluorescence and bio-affinity properties. J. Mater. Chem. 2004, 14, 1336–1341. [Google Scholar] [CrossRef]
- He, H.; Xie, M.; Ding, Y.; Yu, X.-F. Synthesis of Fe3O4@LaF3:Ce,Tb nanocomposites with bright fluorescence and strong magnetism. Appl. Surf. Sci. 2009, 255, 4623–4626. [Google Scholar] [CrossRef]
- Cui, X.; Mathe, D.; Kovács, N.; Horváth, I.; Jauregui-Osoro, M.; de Rosales, R.T.M.; Mullen, G.E.D.; Wong, W.; Yan, Y.; Krüger, D.; et al. Synthesis, Characterization, and Application of Core-Shell Co0.16Fe2.84O4@NaYF4(Yb, Er) and Fe3O4@NaYF4(Yb, Tm) Nanoparticle as Trimodal (MRI, PET/SPECT, and Optical) Imaging Agents. Bioconjug. Chem. 2016, 27, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Sun, L.-D.; Zhang, Y.-W.; Yan, C. Superparamagnetic and upconversion emitting Fe3O4/NaYF4:Yb,Er hetero-nanoparticles via a crosslinker anchoring strategy. Chem. Commun. 2010, 46, 5731. [Google Scholar] [CrossRef]
- Flores-Martinez, N.; Roemer, M.; Gam-Derouich, S.; Beaunier, P.; Habrovsky, D.; Ammar, S.; Flores, N.; Hrabovsky, D. Magnetic Traits in CoO-Core@CoFe2O4 -shell Like Nanoparticles. ChemNanoMat 2019, 5, 514–524. [Google Scholar] [CrossRef]
- Franceschin, G.; Gaudisson, T.; Menguy, N.; Dodrill, B.C.; Yaacoub, N.; Grenèche, J.-M.; Valenzuela, R.; Ammar, S. Exchange-Biased Fe3−xO4-CoO Granular Composites of Different Morphologies Prepared by Seed-Mediated Growth in Polyol: From Core–Shell to Multicore Embedded Structures. Part. Part. Syst. Charact. 2018, 35, 1800104. [Google Scholar] [CrossRef]
- Flores-Martinez, N.; Franceschin, G.; Gaudisson, T.; Beaunier, P.; Yaacoub, N.; Grenèche, J.-M.; Valenzuela, R.; Ammar, S. Giant Exchange-Bias in Polyol-Made CoFe2O4-CoO Core–Shell Like Nanoparticles. Part. Part. Syst. Charact. 2018, 35, 1800290. [Google Scholar] [CrossRef]
- Mnasri, W.; Tahar, L.B.; Boissière, M.; Haidar, D.A.; Ammar, S. The first one-pot synthesis of undoped and Eu doped β-NaYF4 nanocrystals and their evaluation as efficient dyes for nanomedicine. Mater. Sci. Eng. C 2019, 1, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-N.; Wei, C.; Zhu, Z.-Z.; Hou, Y.; Venkatraman, S.S.; Xu, Z.J.; Venkatraman, S.S. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23, 037503. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H. MAUD a friendly Java program for material analysis using diffraction. Newsl. CPD 1999, 21, 14–15. [Google Scholar]
- Arosio, P.; Thévenot, J.; Orlando, T.; Orsini, F.; Corti, M.; Mariani, M.; Bordonali, L.; Innocenti, C.; Sangregorio, C.; Oliveira, H.; et al. Hybrid iron oxide-copolymer micelles and vesicles as contrast agents for MRI: Impact of the nanostructure on the relaxometric properties. J. Mater. Chem. B 2013, 1, 5317. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, T.; Costa, I.; Marinho, V.; Carvalho, V.; Uchôa, K.; Ayres, C.; Teixeira, S.; Vasconcelos, D.F.P. Human foreskin fibroblasts: From waste bag to important biomedical applications. J. Clin. Urol. 2018, 11, 385–394. [Google Scholar] [CrossRef]
- Pasqua, L.; De Napoli, I.E.; De Santo, M.; Greco, M.; Catizzone, E.; Lombardo, D.; Montera, G.; Comandè, A.; Nigro, A.; Morelli, C.; et al. Mesoporous silica-based hybrid materials for bone-specific drug delivery. Nanoscale Adv. 2019, 1, 3269–3278. [Google Scholar] [CrossRef] [Green Version]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.; Tamanoi, F.; Zink, J.I. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-S.; Hsu, Y.-C.; Liao, H.-T.; Yen, F.-S.; Wang, C.-Y.; Hsu, C.-T. Characterization and biocompatibility of chestnut shell fiber-based composites with polyester. J. Appl. Polym. Sci. 2014, 131, 40730. [Google Scholar] [CrossRef]
- Smithmyer, M.E.; Sawicki, L.A.; Kloxin, A.M. Hydrogel scaffolds as in vitro models to study fibroblast activation in wound healing and disease. Biomater Sci. 2014, 2, 634–650. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-S.; Bertrand, V.; Bozukova, D.; Pagnoulle, C.; Labrugère, C.; De Pauw, E.; De Pauw-Gillet, M.-C.; Durrieu, M.-C. RGD Surface Functionalization of the Hydrophilic Acrylic Intraocular Lens Material to Control Posterior Capsular Opacification. PLoS ONE 2014, 9, e114973. [Google Scholar] [CrossRef] [Green Version]
- Spirou, S.V.; Lima, S.A.C.; Bouziotis, P.; Vranješ-Djurić, S.; Efthimiadou, E.Κ.; Laurenzana, A.; Barbosa, A.I.; Garcia-Alonso, I.; Jones, C.; Jankovic, D.; et al. Recommendations for In Vitro and In Vivo Testing of Magnetic Nanoparticle Hyperthermia Combined with Radiation Therapy. Nanomaterials 2018, 8, 306. [Google Scholar] [CrossRef] [Green Version]
- Basti, H.; Ben Tahar, L.; Smiri, L.; Herbst, F.; Vaulay, M.-J.; Chau, F.; Ammar, S.; Benderbous, S. Catechol derivatives-coated Fe3O4 and γ-Fe2O3 nanoparticles as potential MRI contrast agents. J. Colloid Interface Sci. 2010, 341, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.L.; Berret, J.; Fresnais, J.; Gossuin, Y.; Sandre, O. A universal scaling law to predict the efficiency of magnetic nanoparticles as MRI T(2)-contrast agents. Adv. Healthcare Mater. 2012, 1, 502–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Y.; Sherwood, J.A.; Sun, Z. Magnetic iron oxide nanoparticles asT1contrast agents for magnetic resonance imaging. J. Mater. Chem. C 2018, 6, 1280–1290. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Zhang, Y.; Sheng, G. Controlled synthesis and tunable luminescence of NaYF4:Eu3+. J. Rare Earths 2010, 28, 222–224. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Wang, C.; Chang, J.; Shi, H.; Lin, J. Photoluminescence properties of LaF3:Eu3+ nanoparticles prepared by refluxing method. J. Rare Earths 2009, 27, 33–37. [Google Scholar] [CrossRef]
- Groman, E.V.; Bouchard, J.C.; Reinhardt, C.P.; Vaccaro, D.E. Ultrasmall Mixed Ferrite Colloids as Multidimensional Magnetic Resonance Imaging, Cell Labeling, and Cell Sorting Agents. Bioconjug. Chem. 2007, 18, 1763–1771. [Google Scholar] [CrossRef]











| Samples | Fluoride Phase | Oxide Phase | <DTEM> (nm) | ||||
|---|---|---|---|---|---|---|---|
| a,c (Å) | <LXRD> (nm) | <ε> | a (Å) | <LXRD> (nm) | <ε> | ||
| β-NaY0.8Eu0.2F4 | 5.993(5) 3.530(5) | 66 | 10 × 10−4 | - | - | - | 67(7) |
| γ-Fe2O3 | - | - | - | 8.375(7) | 9 | 18 × 10−4 | 9(2) |
| β-NaY0.8Eu0.2F4@γ-Fe2O3 | 5.992(5) 3.526(5) | 72 | 22 × 10−4 | 8.378(7) | 8 | 22 × 10−4 | 89(9) |
| Samples | [Fe] a mMm | [Fe] b mM | r1 s−1mM−1 | r2 s−1mM−1 | <DDLS> c nm | PDI c |
|---|---|---|---|---|---|---|
| γ-Fe2O3 | 23.1 | 19.0 | 21.9 | 134.1 | 66 | 0.28 |
| β-NaY0.8Eu0.2F4 @ γ-Fe2O3 | 6.6 | 7.7 | 12.7 | 34.6 | 150 | 0.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mnasri, W.; Ben Tahar, L.; Beaunier, P.; Abi Haidar, D.; Boissière, M.; Sandre, O.; Ammar, S. Polyol-Made Luminescent and Superparamagnetic β-NaY0.8Eu0.2F4@γ-Fe2O3 Core-Satellites Nanoparticles for Dual Magnetic Resonance and Optical Imaging. Nanomaterials 2020, 10, 393. https://doi.org/10.3390/nano10020393
Mnasri W, Ben Tahar L, Beaunier P, Abi Haidar D, Boissière M, Sandre O, Ammar S. Polyol-Made Luminescent and Superparamagnetic β-NaY0.8Eu0.2F4@γ-Fe2O3 Core-Satellites Nanoparticles for Dual Magnetic Resonance and Optical Imaging. Nanomaterials. 2020; 10(2):393. https://doi.org/10.3390/nano10020393
Chicago/Turabian StyleMnasri, Walid, Lotfi Ben Tahar, Patricia Beaunier, Darine Abi Haidar, Michel Boissière, Olivier Sandre, and Souad Ammar. 2020. "Polyol-Made Luminescent and Superparamagnetic β-NaY0.8Eu0.2F4@γ-Fe2O3 Core-Satellites Nanoparticles for Dual Magnetic Resonance and Optical Imaging" Nanomaterials 10, no. 2: 393. https://doi.org/10.3390/nano10020393