Earth Abundant Oxidation Catalysts for Removal of Contaminants of Emerging Concern from Wastewater: Homogeneous Catalytic Screening of Monomeric Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. X-ray Crystal Structures
2.3. Acid Decomplexation Studies
2.4. Cyclic Voltammetry
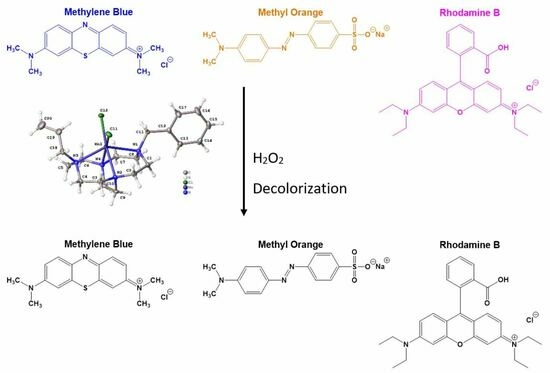
2.5. Dye-Bleaching Studies
3. Experimental
3.1. General
3.2. Synthesis
3.2.1. Synthesis of Novel Ligands and Precursors
3.2.2. Synthesis of Novel Metal Complexes
General Procedure for Complexation of CuCl2, MnCl2, or FeCl2
3.3. Characterization
3.3.1. Acid Decomplexation Studies
3.3.2. X-ray Crystallography Studies
3.4. Dye-Bleaching Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Schäfer, F.P.; Schmidt, W.; Volze, J. Organic dye solution laser. Appl. Phys. Lett. 1966, 9, 306–309. [Google Scholar] [CrossRef]
- Mustroph, H.; Stollenwerk, M.; Bressau, V. Current Developments in Optical Data Storage with Organic Dyes. Angew. Chem. 2006, 45, 2016–2035. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total. Environ. 2020, 717, 137–222. [Google Scholar] [CrossRef]
- Epling, G.A.; Lin, C. Photoassisted bleaching of dyes utilizing TiO2 and visible light. Chemosphere 2002, 46, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, R.; Guo, Y.; Xu, L.; Liu, J. Effects of chloride ions on bleaching of azo dyes by Co2+/oxone regent: Kinetic analysis. J. Hazard. Mater. 2011, 190, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Neves, C.M.B.; Simões, M.M.Q.; Neves, M.D.G.P.M.S.; Cocco, G.S.; Sanjust, E. Immobilized Lignin Peroxidase-Like Metalloporphyrins as Reusable Catalysts in Oxidative Bleaching of Industrial Dyes. Molecules 2016, 21, 964. [Google Scholar] [CrossRef]
- Santos, M.C.; Antonin, V.S.; Souza, F.M.; Aveiro, L.R.; Pinheiro, V.S.; Gentil, T.C.; Lima, T.S.; Moura, J.P.C.; Silva, C.R.; Lucchetti, L.E.B.; et al. Decontamination of wastewater containing contaminants of emerging concern by electrooxidation and Fenton-based processes—A review on the relevance of materials and methods. Chemosphere 2022, 307, 135763. [Google Scholar] [CrossRef]
- Kiselev, V.M.; Evstrop’ev, S.K.; Starodubtsev, A.M. Photocatalytic degradation and sorption of methylene blue on the surface of metal oxides in aqueous solutions of the dye. Opt. Spectrosc. 2017, 123, 809–815. [Google Scholar] [CrossRef]
- da Rocha, M.G.; Nakagaki, S.; Ucoski, G.M.; Wypych, F.; Machado, G.S. Comparison between catalytic activities of two zinc layered hydroxide salts in brilliant green organic dye bleaching. J. Colloid Interface Sci. 2019, 541, 425–433. [Google Scholar] [CrossRef]
- Zhang, Y.H.G.; Zhang, G. A novel mixed-phase TiO2/kaolinite composites and their photocatalytic activity for degradation of organic contaminants. Chem. Eng. J. 2011, 172, 936–943. [Google Scholar] [CrossRef]
- Hubin, T.J.; McCormick, J.M.; Collinson, S.R.; Buchalova, M.; Perkins, C.M.; Alcock, N.W.; Kahol, P.K.; Raghunathan, A.; Busch, D.H. New iron(II) and manganese(II) complexes of two ultra-rigid, cross-bridged tetraazamacrocycles for catalysis and biomimicry. J. Am. Chem. Soc. 2000, 122, 2512–2522. [Google Scholar] [CrossRef]
- Hubin, T.J.; McCormick, J.M.; Collinson, S.R.; Alcock, N.W.; Clase, H.J.; Busch, D.H. Synthesis and X-ray crystal structures of iron(II) and manganese(II) complexes of unsubstituted and benzyl substituted cross-bridged tetraazamacrocycles. Inorg. Chim. Acta 2003, 346, 76–86. [Google Scholar] [CrossRef]
- Busch, D.H.; Collinson, S.R.; Hubin, T. Catalysts and Methods for Catalytic Oxidation. U.S. Patent 6,906,189, 14 June 2005. [Google Scholar]
- Yin, G.; Danby, A.M.; Kitko, D.; Carter, J.D.; Scheper, W.M.; Busch, D.H. Olefin Epoxidation by Alkyl Hydroperoxide with a Novel Cross-Bridged Cyclam Manganese Complex: Demonstration of Oxygenation by Two Distinct Reactive Intermediates. Inorg. Chem. 2007, 46, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.C.; Danby, A.M.; Kitko, D.; Carter, J.D.; Scheper, W.M.; Busch, D.H. Oxidative reactivity difference among the metal oxo and metal hydroxo moieties: pH dependent hydrogen abstraction by a manganese(IV) complex having two hydroxide ligands. J. Am. Chem. Soc. 2008, 130, 16245–16253. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Geiger, R.A.; Yin, G.; Busch, D.H.; Jackson, T.A. Oxo- and Hydroxomanganese(IV) Adducts: A Comparative Spectroscopic and Computational Study. Inorg. Chem. 2010, 49, 7530–7535. [Google Scholar] [CrossRef]
- Dong, L.; Wang, Y.; Lu, Y.; Chen, Z.; Mei, F.; Xiong, H.; Yin, G. Lewis-Acid-Promoted Stoichiometric and Catalytic Oxidations by Manganese Complexes Having Cross-Bridged Cyclam Ligand: A Comprehensive Study. Inorg. Chem. 2013, 52, 5418–5427. [Google Scholar] [CrossRef]
- Tan, P.; Kwong, H.-K.; Lau, T.-C. Catalytic oxidation of water and alcohols by a robust iron(iii) complex bearing a cross-bridged cyclam ligand. Chem. Commun. 2015, 51, 12189–12192. [Google Scholar] [CrossRef]
- Annunziata, A.; Esposito, R.; Gatto, G.; Cucciolito, M.E.; Tuzi, A.; Macchioni, A.; Ruffo, F. Iron(III) Complexes with Cross-Bridged Cyclams: Synthesis and Use in Alcohol and Water Oxidation Catalysis. Eur. J. Inorg. Chem. 2018, 2018, 3304–3311. [Google Scholar] [CrossRef]
- Matz, D.L.; Jones, D.G.; Roewe, K.D.; Gorbet, M.J.; Zhang, Z.; Chen, Z.Q.; Prior, T.J.; Archibald, S.J.; Yin, G.C.; Hubin, T.J. Synthesis, structural studies, kinetic stability, and oxidation catalysis of the late first row transition metal complexes of 4,10-dimethyl-1,4,7,10-tetraazabicyclo [6.5.2]pentadecane. Dalton Trans. 2015, 44, 12210–12224. [Google Scholar] [CrossRef]
- Jones, D.G.; Wilson, K.R.; Cannon-Smith, D.J.; Shircliff, A.D.; Zhang, Z.; Chen, Z.; Prior, T.J.; Yin, T.J.; Hubin, T.J. Synthesis, structural studies, and oxidation catalysis of the late-first-row-transition-metal complexes of a 2-pyridylmethyl pendant-armed ethylene cross-bridged cyclam. Inorg. Chem. 2015, 54, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Odendaal, A.Y.; Fiamengo, A.L.; Ferdani, R.; Wadas, T.J.; Hill, D.C.; Peng, Y.; Heroux, K.J.; Golen, J.A.; Rheingold, A.L.; Anderson, C.J.; et al. Isomeric Trimethylene and Ethylene Pendant-armed Cross-bridged Tetraazamacrocycles and in Vitro/in Vivo Comparisions of their Copper(II) Complexes. Inorg. Chem. 2011, 50, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wuest, M.; Weisman, G.R.; Wong, E.H.; Reed, D.P.; Boswell, C.A.; Motekaitis, R.; Martell, A.E.; Welch, M.J.; Anderson, C.J. Radiolabeling and in vivo behavior of copper-64-labeled cross-bridged cyclam ligands. J. Med. Chem. 2002, 45, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Boswell, C.A.; Sun, X.; Niu, W.; Weisman, G.R.; Wong, E.H.; Rheingold, A.L.; Anderson, C.J. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J. Med. Chem. 2004, 47, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Heroux, K.J.; Woodin, K.S.; Tranchemontagne, D.J.; Widger, P.C.B.; Southwick, E.; Wong, E.H.; Weisman, G.R.; Tomellini, S.A.; Wadas, T.J.; Anderson, C.J.; et al. The long and short of it: The influence of N-carboxyethyl versus N-carboxymethyl pendant arms on in vitro and in vivo behavior of copper complexes of cross-bridged tetraamine macrocycles. Dalton Trans. 2007, 21, 2150–2162. [Google Scholar] [CrossRef]
- Sprague, J.E.; Peng, Y.; Fiamengo, A.L.; Woodin, K.S.; Southwick, E.A.; Weisman, G.R.; Wong, E.H.; Golen, J.A.; Rheingold, A.L.; Anderson, C.J. Synthesis, Characterization and In Vivo Studies of Cu(II)-64-Labeled Cross-Bridged Tetraazamacrocycle-amide Complexes as Models of Peptide Conjugate Imaging Agents. J. Med. Chem. 2007, 50, 2527–2535. [Google Scholar] [CrossRef]
- Anderson, C.J.; Wadas, T.J.; Wong, E.H.; Weisman, G.R. Cross-bridged Macrocyclic Chelators for Stable Complexation of Copper Radionuclides for PET Imaging. Q. J. Nucl. Med. Mol. Imaging 2008, 52, 185–192. [Google Scholar]
- Ferdani, R.; Stigers, D.J.; Fiamengo, A.L.; Wei, L.; Li, B.T.Y.; Golen, J.A.; Rheingold, A.L.; Wiesman, G.R.; Wong, E.H.; Anderson, C.J. Syntheis, Cu(II) complexation, 64Cu-labeling and biological evaluation of cross-bridged cyclam chelators with phosphonate pendant arms. Dalton Trans. 2012, 41, 1938–1950. [Google Scholar] [CrossRef]
- Burke, B.P.; Miranda, C.S.; Lee, R.E.; Renard, I.; Nigam, S.; Clemente, G.S.; D’Huys, T.; Ruest, T.; Domarkas, J.; Thompson, J.A.; et al. Cu PET Imaging of the CXCR4 Chemokine Receptor Using a Cross-Bridged Cyclam Bis-Tetraazamacrocyclic Antagonist. J. Nucl. Med. 2020, 61, 123–128. [Google Scholar] [CrossRef]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef]
- Khan, A.; Nicholson, G.; Greenman, J.; Madden, L.; McRobbie, G.; Pannecouque, C.; De Clercq, E.; Ullom, R.; Maples, D.L.; Maples, R.D.; et al. Binding Optimization through Coordination Chemistry: CXCR4 Chemokine Receptor Antagonists from Ultrarigid Metal Complexes. J. Am. Chem. Soc. 2009, 131, 3416–3417. [Google Scholar] [CrossRef] [PubMed]
- Maples, R.D.; Cain, A.N.; Burke, B.P.; Silversides, J.D.; Mewis, R.; D’huys, T.; Schols, D.; Linder, D.P.; Archibald, S.J.; Hubin, T.J. Aspartate-based CXCR4 chemokine receptor binding of cross-bridged tetraazamacrocyclic copper(II) and zinc(II) complexes. Chem. A Eur. J. 2016, 22, 12916–12930. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.; Mondal, S.; Allen, M.B.; Garcia, L.; Krause, J.A.; Oliver, A.G.; Prior, T.J.; Hubin, T.J. Synthesis and Characterization of Late Transition Metal Complexes of Mono-Acetate Pendant Armed Ethylene Cross-Bridged Tetraazamacrocycles with Promise as Oxidation Catalysts for Dye Bleaching. Molecules 2023, 28, 232. [Google Scholar] [CrossRef] [PubMed]
- Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Ganesan, V.; Yoon, S. Direct Heterogenization of Salphen Coordination Complexes to Porous Organic Polymers: Catalysts for Ring-Expansion Carbonylation of Epoxides. Inorg. Chem. 2020, 59, 2881–2889. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, T.; Lin, J.; Lu, J.; Lin, Z.; Cao, R. Homochiral NIckel Coordination Polymers Based on Salen(Ni) Metalloligands: Synthesis, Structure, and Catalytic Alkene Epoxidation. Inorg. Chem. 2011, 50, 2191–2198. [Google Scholar] [CrossRef]
- Yuan, G.; Jiang, H.; Zhang, L.; Liu, Y.; Cui, Y. Metallosalen-based crystalline pourous materials: Synthesis and property. Coord. Chem. Rev. 2019, 378, 483–499. [Google Scholar] [CrossRef]
- Liu, T.-T.; Liang, J.; Xu, R.; Huang, Y.-B.; Cao, R. Salen-Co(III) insertion in multivariate cationic metal-organic frameworks for the enhanced cycloaddition reaction of carbon dioxide. Chem. Commun. 2019, 55, 4063–4066. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef]
- Friedel, C.; Crafts, J.M. Sur une nouvelle méthode générale de synthèse d’hydrocarbures, d’acétones, etc. Compte. Rend. 1877, 84, 1392. [Google Scholar]
- Begni, F.; Gullo, F.; Paul, G.; Rea, R.; Ferrari, M.-C.; Mangano, E.; Cossi, M.; Gatti, G.; Marchese, L. Optimization of the Friedel–Crafts Alkylation for the Synthesis of Hyper-Cross-Linked Polymers. ACS Appl. Polym. Mater. 2022, 4, 5281–5286. [Google Scholar] [CrossRef]
- Leeuwen, P.W.N.M. Alkene Polymerisation. In Homogeneous Catalysis, 1st ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 191–228. [Google Scholar] [CrossRef]
- Zuo, X.; Mosha, D.; Archibald, S.J.; McCasland, A.K.; Hassan, A.M.; Givens, R.S.; Busch, D.H. Towards the soil poultice and a new separations methodology: Rebinding of macrocyclic metal complexes to molecularly imprinted polymers specifically templated via noncovalent interactions. J. Coord. Chem. 2005, 58, 21–39. [Google Scholar] [CrossRef]
- Weisman, G.R.; Wong, E.H.; Hill, D.C.; Rogers, M.E.; Reed, D.P.; Calabrese, J.C. Synthesis and transition-metal complexes of new cross-bridged tetraamine ligands. Chem. Commun. 1996, 8, 947–948. [Google Scholar] [CrossRef]
- Khan, M.O.F.; Keiser, J.; Amoyay, P.N.A.; Hossain, M.F.; Vargas, M.; Le, J.G.; Simpson, N.C.; Roewe, K.D.; Carder Freeman, T.N.; Hasley, T.R.; et al. Discovery of Antischistosomal Drug Leads Based on Tetraazamacrocyclic Derivatives and Their Metal Complexes. Antimicr. Agents Chemoth. 2016, 60, 5331–5336. [Google Scholar] [CrossRef] [PubMed]
- Rohovec, J.; Gyepes, R.; Cisarova, I.; Rudovsky, J.; Lukes, I. Nucleophilic reactivity of perhydro-3,6,9,12-tetraazacyclopenteno[1,3-f,g]acenaphthylene. A unified approach to N-monosubstituted and N,N″-disubstituted cyclen derivatives. Tetrahedron Lett. 2000, 41, 1249–1253. [Google Scholar] [CrossRef]
- Le Baccon, M.; Chuburu, F.; Toupet, L.; Handel, H.; Soibinet, M.; Deschamps-Olivier, I.; Barbier, J.-P.; Aplincourt, M. Bis-aminals: Efficient tools for bis-macrocycle synthesis. New J. Chem. 2001, 25, 1168–1174. [Google Scholar] [CrossRef]
- Hubin, T.J.; McCormick, J.M.; Collinson, S.R.; Alcock, N.W.; Busch, D.H. Ultra rigid cross-bridged tetraazamacrocycles as ligands—The challenge and the solution. Chem. Commun. 1998, 16, 1675–1676. [Google Scholar] [CrossRef]
- Hubin, T.J.; Walker, A.N.; Davilla, D.J.; Carder Freeman, T.N.; Epley, B.M.; Hasley, T.R.; Amoyaw, P.N.A.; Jain, S.; Archibald, S.J.; Prior, T.J.; et al. Tetraazamacrocyclic derivatives and their metal complexes as antileishmanial leads. Polyhedron 2019, 163, 42–53. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Hubin, T.J.; McCormick, J.M.; Alcock, N.W.; Clase, H.J.; Busch, D.H. Crystallographic characterization of stepwise changes in ligand conformations as their internal topology changes and two novel cross-bridged tetraazamacrocyclic Copper(II) complexes. Inorg. Chem. 1999, 38, 4435–4446. [Google Scholar] [CrossRef]
- AlHaddad, N.; Lelong, E.; Suh, J.-M.; Cordier, M.; Lim, M.H.; Royal, G.; Platas-Iglesias, C.; Bernard, H.; Tripier, R. Copper(II) and zinc(II) complexation with N-ethylene hydroxycyclams and consequences on the macrocyclic backbone configuration. Dalton Trans. 2022, 51, 8640–8656. [Google Scholar] [CrossRef] [PubMed]
- Woodin, K.S.; Heroux, K.J.; Boswell, C.A.; Wong, E.H.; Weisman, G.R.; Niu, W.; Tomellini, S.A.; Anderson, C.J.; Zakharov, L.N.; Rheingold, A.L. Kinetic inertness and electrochemical behavior of Cu(II) tetraazamacrocyclic complexes: Possible implications for in vivo stability. Eur. J. Inorg. Chem. 2005, 7, 4829–4833. [Google Scholar] [CrossRef]
- Hubin, T.J.; Alcock, N.W.; Morton, M.D.; Busch, D.H. Synthesis, structure, and stability in acid of copper(II) and zinc(II) complexes of cross-bridged tetraazamacrocycles. Inorg. Chim. Acta 2003, 348, 33–40. [Google Scholar] [CrossRef]
- Walker, A.N.; Ayala, M.A.; Mondal, S.; Bergagnini, M.C.; Bui, P.J.D.; Chidester, S.N.; Doeden, C.I.; Esjornson, L.; Sweany, B.R.; Garcia, L.; et al. A Bridge Too Far? Comparison of Transition Metal Complexes of Dibenzyltetraazamacrocycles with and without Ethylene Cross-Bridges: X-ray Crystal Structures, Kinetic Stability, and Electronic Properties. Molecules 2023, 28, 895. [Google Scholar] [CrossRef]
- Busch, D.H. The Compleat Coordination Chemistery--One Practioner’s Perspective. Chem. Rev. 1993, 93, 847–860. [Google Scholar] [CrossRef]
- Schubert, U.S.; Andres, P.R. New functional polymers and materials based on 2,2′:6′2″-terpyridine metal complexes. Adv. Mater. 2004, 16, 1043–1068. [Google Scholar]
- Park, J.; Pasupathy, A.N.; Goldsmith, J.I.; Chang, C.; Yaish, Y.; Petta, J.R.; Rinkoski, M.; Sethna, J.P.; Abruna, H.D.; McEuen, P.L.; et al. Coulomb blockade and the Kondo effect in single-atom transistors. Nature 2002, 417, 722–725. [Google Scholar] [CrossRef]
- Ledwon, P.; Ovsiannikova, D.; Jarosz, T.; Gogoc, S.; Nitschke, P.; Domagala, W. Insight into the properties and redox states of n-dopable conjugated polymers based on naphtalene diimide units. Electrochim. Acta 2019, 307, 525–535. [Google Scholar] [CrossRef]
- Kulthanan, K.; Nuchkull, P.; Varothai, S. The pH of water from various sources: An overview for recommendation for patients with atopic dermatitis. Asia Pacific. Allergy 2013, 3, 155–160. [Google Scholar] [CrossRef]
- Bayabil, H.K.; Teshome, T.; Li, Y.C. Emerging Contaminants in Soil and Water. Front. Environ. Sci. 2022, 10, 873499. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef] [PubMed]
- Niang, M.; Reichard, J.F.; Maier, A.; Talaska, G.; Ying, J.; Domingo, J.S.; Varughese, E.; Boczek, L.; Huff, E.; Reponen, T. Ciprofloxacin- and azithromycin-resistant bacteria in a wastewater treatment plant. J. Occup. Environ. Hyg. 2023, 20, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Mangalgiri, K.P.; Blaney, L. Elucidating the Stimulatory and Inhibitory Effects of Dissolved Organic Matter from Poultry Litter on Photodegradation of Antibiotics. Environ. Sci. Technol. 2017, 51, 12310–12320. [Google Scholar] [CrossRef] [PubMed]
- Mangalgiri, K.P.; Timko, S.A.; Gonsior, M.; Blaney, L. PARAFAC Modeling of Irradiation- and Oxidation-Induced Changes in Fluorescent Dissolved Organic Matter Extracted from Poultry Litter. Environ. Sci. Technol. 2017, 51, 8036–8047. [Google Scholar] [CrossRef]
- Mangalgiri, K.P.; Patton, S.; Wu, L.; Xu, S.; Ishida, K.P.; Liu, H. Optimizing Potable Water Reuse Systems: Chloramines or Hydrogen Peroxide for UV-Based Advanced Oxidation Process? Environ. Sci. Technol. 2019, 53, 13323–13331. [Google Scholar] [CrossRef]
- Xu, A.; Xiong, H.; Yin, G. Decolorization of Dye Pollutants by Manganse Complexes with Rigid Cross-Bridged Cyclam Ligands and Its Mechanistic Investigations. J. Phys. Chem. A 2009, 113, 12243–12248. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.X. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]











| Identification code | [MnL1Cl2] | [MnL3Cl2] | [FeL3Cl2] | L8-precursor | [MnL9Cl2] | [MnL11Cl2] | [FeL11Cl2]. 2MeCN |
| Empirical formula | C10H22Cl2MnN4 | C14H28Cl2MnN4 | C14H28Cl2FeN4 | C20H34I2N4O2 | C16H30Cl2MnN4 | C20H32Cl2MnN4 | C22H35Cl2FeN5 |
| Formula weight | 324.15 | 378.24 | 379.15 | 616.31 | 404.28 | 454.33 | 496.30 |
| Temperature/K | 120 | 120 | 120 | 150 | 120 | 120 | 120 |
| Crystal system | monoclinic | orthorhombic | orthorhombic | monoclinic | triclinic | monoclinic | orthorhombic |
| Space group | Cc | P212121 | P212121 | P21/n | P-1 | P21/c | Pnma |
| a/Å | 12.3081(13) | 7.6390(4) | 7.6198(5) | 9.5389(10) | 8.5540(11) | 8.4238(4) | 14.4316(7) |
| b/Å | 10.1119(13) | 12.0399(6) | 11.9637(8) | 16.9155(17) | 13.2482(17) | 13.5803(6) | 8.9132(5) |
| c/Å | 11.2029(12) | 18.6843(10) | 18.6306(14) | 14.7768(14) | 17.920(2) | 18.6065(8) | 19.5405(10) |
| α/° | 90 | 90 | 90 | 90 | 107.492(2) | 90 | 90 |
| β/° | 96.578(2) | 90 | 90 | 91.479(4) | 95.600(2) | 98.507(2) | 90 |
| γ/° | 90 | 90 | 90 | 90 | 97.994(2) | 90 | 90 |
| Volume/Å3 | 1385.1(3) | 1718.45(15) | 1698.4(2) | 2383.5(4) | 1897.0(4) | 2105.12(16) | 2513.5(2) |
| Z | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| ρcalc/gcm−3 | 1.554 | 1.462 | 1.483 | 1.717 | 1.416 | 1.434 | 1.311 |
| μ/mm−1 | 1.324 | 1.078 | 1.202 | 3.350 | 0.982 | 0.894 | 0.831 |
| F(000) | 676.0 | 796.0 | 800.0 | 1216.0 | 852.0 | 956.0 | 1048.0 |
| Crystal size/mm3 | 0.355 × 0.247 × 0.142 | 0.213 × 0.15 × 0.078 | 0.287 × 0.134 × 0.127 | 0.05 × 0.02 × 0.01 | 0.253 × 0.154 × 0.05 | 0.253 × 0.169 × 0.078 | 0.216 × 0.169 × 0.088 |
| Radiation/Å | MoKα, 0.71073 | MoKα, 0.71073 | MoKα, 0.71073 | Synchrotron, 0.7749 | MoKα, 0.71073 | MoKα, 0.71073 | MoKα, 0.71073 |
| 2Θ range for data collection/° | 5.228 to 56.576 | 4.024 to 56.618 | 4.046 to 56.798 | 3.992 to 52.228 | 2.41 to 56.584 | 3.728 to 56.632 | 3.508 to 56.726 |
| Index ranges | −16 ≤ h ≤ 14 −13 ≤ k ≤ 13 −14 ≤ l ≤ 14 | −10 ≤ h ≤ 10 −16 ≤ k ≤ 16 −24 ≤ l ≤ 24 | −10 ≤ h ≤ 9 −15 ≤ k ≤ 15 −24 ≤ l ≤ 24 | −10 ≤ h ≤ 10 −19 ≤ k ≤ 19 −16 ≤ l ≤ 16 | −11 ≤ h ≤ 11 −17 ≤ k ≤ 17 −23 ≤ l ≤ 23 | −11 ≤ h ≤ 11 −18 ≤ k ≤ 18 −24 ≤ l ≤ 24 | −19 ≤ h ≤ 19 −11 ≤ k ≤ 11 −26 ≤ l ≤ 26 |
| Reflections collected | 13204 | 31559 | 41891 | 37933 | 29491 | 49812 | 46814 |
| Independent reflections | 3268 | 4259 | 4239 | 3652 | 9415 | 5246 | 3344 |
| Rint | 0.0178 | 0.0520 | 0.0400 | 0.1904 | 0.0370 | 0.0340 | 0.0444 |
| Data/restraints/parameters | 3268/2/167 | 4259/0/192 | 4239/0/192 | 3652/0/269 | 9415/41/437 | 5246/0/244 | 3344/235/201 |
| Goodness-of-fit on F2 | 1.042 | 1.180 | 1.064 | 1.037 | 1.005 | 1.089 | 1.031 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0131 wR2 = 0.0319 | R1 = 0.0400 wR2 = 0.0740 | R1 = 0.0385 wR2 = 0.0920 | R1 = 0.0617 wR2 = 0.1579 | R1 = 0.0307 wR2 = 0.0591 | R1 = 0.0264 wR2 = 0.0603 | R1 = 0.0284 wR2 = 0.0652 |
| Final R indexes [all data] | R1 = 0.0133 wR2 = 0.0320 | R1 = 0.0445 wR2 = 0.0751 | R1 = 0.0401 wR2 = 0.0931 | R1 = 0.0820 wR2 = 0.1757 | R1 = 0.0499 wR2 = 0.0645 | R1 = 0.0294 wR2 = 0.0615 | R1 = 0.0372 wR2 = 0.0693 |
| Largest diff. peak/hole/e Å−3 | 0.18/−0.12 | 0.37/−0.52 | 0.93/−0.57 | 1.03/−0.74 | 0.33/−0.51 | 0.39/−0.22 | 0.30/−0.23 |
| Flack parameter | 0.077(10) | 0.32(3) *(racemic twin) | 0.47(3) *(racemic twin) | none | none | none | none |
| Structure | M-N1/Å M-N3/Å | M-N2/Å M-N4/Å | N1-M-N3/° N2-M-N4/° |
|---|---|---|---|
| [Mn(L1)Cl2] | 2.2761(14) 2.2935(13) | 2.3255(14) 2.3041(13) | 142.60(5) 73.49(5) |
| [Mn(L3)Cl2] | 2.392(3) 2.313(3) | 2.302(3) 2.314(3) | 142.48(10) 74.85(10) |
| [Fe(L3)Cl2] | 2.320(3) 2.257(3) | 2.235(4) 2.233(4) | 146.15(12) 77.20(11) |
| [Fe(L5)(OAc)2] Ref: [50] | 2.3024(10) 2.3024(9) | 2.2091(10) 2.2228(11) | 148.84(4) 77.91(4) |
| [Mn(L9)Cl2] | Mn1: 2.3378(13) 2.3242(14) Mn2: 2.3510(14) 2.3786(15) | Mn1: 2.2706(14) 2.2981(13) Mn2: 2.3138(14) 2.2873(14) | Mn1: 144.69(5) 74.86(5) Mn2: 143.88(5) 74.15(5) |
| [Mn(L11)Cl2] | 2.4019(11) 2.3935(11) | 2.3090(11) 2.2834(11) | 143.56(4) 74.09(4) |
| [Fe(L11)Cl2] ˑ 2MeCN | 2.2705(15) 2.262(2) | 2.278(3) 2.169(3) | 148.85(7) 77.38(12) |
| Ligand | Pseudo-First-Order Half-Life for Decomplexation of 1.000 mM Copper Complexes | |||||
|---|---|---|---|---|---|---|
| 30 °C 1 M HCl | 30 °C 5 M HCl | 50 °C 5 M HCl | 70 °C 5 M HCl | 90 °C 5 M HCl | References | |
 Me2Bcyclen Me2Bcyclen | 36 min | <1 min | [55] | |||
 Me2EBC Me2EBC | 7.3 day | 79 min | [22,55] | |||
 H2Bcyclen (L1) H2Bcyclen (L1) | <1 min | [21] | ||||
 H2Bcyclam (L2) H2Bcyclam (L2) | 18.5 d | 5.40 h [This work] | 11.8 min | [54] | ||
 Me1Allyl1Bcyclen (L3) Me1Allyl1Bcyclen (L3) | 25 min | This work | ||||
 Me1Allyl1Bcyclam (L4) Me1Allyl1Bcyclam (L4) | 2.33 h | This work | ||||
 Bn2Bcyclen (L5) Bn2Bcyclen (L5) | 4.2 h | 7.8 min | [33,56] | |||
 Bn2Bcylam (L6) Bn2Bcylam (L6) | 2.38 h | 23.4 min | [33,56] | |||
 Me1Bn1Bcyclen (L7) Me1Bn1Bcyclen (L7) | 24.8 min | This work | ||||
 Me1Bn1Bcyclam (L8) Me1Bn1Bcyclam (L8) | 15.05 h | 1.4 h | This work | |||
 Allyl2Bcyclen (L9) Allyl2Bcyclen (L9) | 51.7 min | This work | ||||
 Allyl2Bcyclam (L10) Allyl2Bcyclam (L10) | 1.86 h | 15 min | This work | |||
| Complex | E1/2 M2+/3+ [V] (∆V [mV]) | E1/2 M3+/4+ [V] (∆V [mV]) | Reference |
|---|---|---|---|
| Fe(Me2Bcyclen)Cl2 | +0.036 (64) | ----- | [12] |
| Mn(Me2Bcyclen)Cl2 | +0.466 (70) | +1.232 (102) | [12] |
| Fe(Me2Bcyclam)Cl2 | +0.110 (63) | ----- | [12] |
| Mn(Me2Bcyclam)Cl2 | +0.585 (61) | 1.343 (65) | [12] |
| [FeL1Cl2] | -0.055 (89) | ----- | [13] |
| [MnL1Cl2] | +0.389 (280) | not accessible in DMF | [13] |
| [FeL2Cl2] | -0.113 (78) | ----- | [13] |
| [MnL2Cl2] | +0.239 (79) | not accessible in DMF | [13] |
| [FeL3Cl2] | +0.094 (82) | ----- | This work |
| [MnL3Cl2] | +0.545 (71) | +1.338 (86) | This work |
| [FeL4Cl2] | +0.165 (96) | ----- | This work |
| [MnL4Cl2] | +0.628 (59) | +1.374 (83) | This work |
| [FeL5Cl2] | +0.071 (85) | ----- | [13] |
| [MnL5Cl2] | +0.400 (65) | not accessible in DMF | [13] |
| [FeL6Cl2] | +0.157 (85) | ----- | [13] |
| [MnL6Cl2] | +0.577 (72) | not accessible in DMF | [13] |
| [FeL7Cl2] | +0.065 (64) | ----- | This work |
| [MnL7Cl2] | +0.550 (76) | +1.355 (82) | This work |
| [FeL8Cl2] | +0.176 (86) | ----- | This work |
| [MnL8Cl2] | +0.609 (93) | +1.404 (92) | This work |
| [FeL9Cl2] | +0.114 (92) | ----- | This work |
| [MnL9Cl2] | +0.572 (68) | +1.385 (79) | This work |
| [FeL10Cl2] | +0.194 (59) | ----- | This work |
| [MnL10Cl2] | +0.616 (72) | +1.422 (83) | This work |
| [FeL11Cl2] | +0.130 (78) | ----- | This work |
| [MnL11Cl2] | +0.558 (72) | +1.390 (73) | This work |
| [FeL12Cl2] | +0.196 (72) | ----- | This work |
| [MnL12Cl2] | +0.619 (85) | +1.433 (92) | This work |
| Catalyst | Average TOF for MB (h−1) (Rank among Top 8) | Average TOF for MO (h−1) (Rank among Top 8) | Average TOF for RhB (h−1) (Rank among Top 8) | Catalyst Rank Based on Average Rank Order for Top 8 Catalysts for All Three Dye TOFs | Highest Redox Potential Observed (Fe3+, Mn3+, or Mn4+) | Half-Life of Cu2+ Complex Under Acid Conditions (Cyclen Complexes in 1 M HCl, 30 °C; Cyclam Complexes in 5 M HCl, 50 °C) |
|---|---|---|---|---|---|---|
| H2O2 only | 0.018 | 0.034 | 0.002 | |||
| FeCl2 only | 0.111 | 0.926 | 0.055 | |||
| MnCl2 only | 0.003 | 0.518 | 0.047 | |||
| [FeL1Cl2] | 0.54 | 1.33 | 0.31 | −0.055 V | <1 min | |
| [MnL1Cl2] | 0.32 | 1.08 | 0.14 | +0.389 V | <1 min | |
| [FeL2Cl2] | 1.21 (7) | 31.35 (3) | 3.62 (1) | 3 (tie) (Avg = 3.67) | −0.113 V | 5.40 h |
| [MnL2Cl2] | 0.27 | 0.147 | 0.18 | +0.239 V | 5.40 h | |
| [FeL3Cl2] | 0.33 | 1.46 | 0.64 | +0.94 V | 25 min | |
| [MnL3Cl2] | 1.67 (5) | 6.42 (8) | 1.104 (7) | 6 (Avg = 6.67) | +1.338 V | 25 min |
| [FeL4Cl2] | 0.35 | 1.26 | 0.63 | +0.165 V | 2.33 h | |
| [MnL4Cl2] | 2.03 (4) | 16.26 (4) | 1.05 (8) | 4 (Avg = 5.33) | +1.374 V | 2.33 h |
| [FeL5Cl2] | 0.13 | 4.08 | 0.21 | +0.071 V | 4.2 h | |
| [MnL5Cl2] | 0.16 | 0.60 | 0.11 | +0.400 V | 4.2 h | |
| [FeL6Cl2] | 0.45 | 3.41 | 0.62 | +0.157 V | 2.38 h | |
| [MnL6Cl2] | 0.74 (8) | 16.14 (5) | 1.27 (5) | 5 (tie) (Avg = 6) | +0.577 V | 2.38 h |
| [FeL7Cl2] | 0.13 | 0.083 | 0.13 | +0.065 V | 24.8 min | |
| [MnL7Cl2] | 2.64 (3) | 8.32 (7) | 2.34 (2) | 3 (tie) (Avg = 3.67) | +1.355 V | 24.8 min |
| [FeL8Cl2] | 0.57 | 2.82 | 1.54 | +0.176 V | 15.05 h | |
| [MnL8Cl2] | 2.09 (3) | 108.9 (1) | 1.46 (4) | 2 (Avg =2.67) | +1.404 V | 15.05 h |
| [FeL9Cl2] | 0.35 | 0.77 | 0.92 | +0.114 V | 51.7 min | |
| [MnL9Cl2] | 0.53 | 3.13 | 0.35 | +1.385 V | 51.7 min | |
| [FeL10Cl2] | 0.171 | 2.2 | 0.35 | +0.194 V | 1.86 h | |
| [MnL10Cl2] | 1.23 (6) | 9.40 (6) | 1.16 (6) | 5 (tie) (Avg = 6) | +1.422 V | 1.86 h |
| [FeL11Cl2] | 0.19 | 0.43 | 0.26 | +0.130 V | n/a | |
| [MnL11Cl2] | 0.18 | 0.86 | 0.15 | +1.390 V | n/a | |
| [FeL12Cl2] | 0.25 | 1.26 | 0.70 | +0.196 V | n/a | |
| [MnL12Cl2] | 5.87 (1) | 36.49 (2) | 1.61 (3) | 1 (Avg = 2.0) | +1.433 V | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, L.; Koper, M.R.; Mondal, S.; Priddle, J.T.; Truong, W.A.; Allbritton, E.M.A.; McAdoo, A.G.; Cannon-Smith, D.J.; Funwie, N.L.; Hoang, T.; et al. Earth Abundant Oxidation Catalysts for Removal of Contaminants of Emerging Concern from Wastewater: Homogeneous Catalytic Screening of Monomeric Complexes. Molecules 2023, 28, 6466. https://doi.org/10.3390/molecules28186466
Garcia L, Koper MR, Mondal S, Priddle JT, Truong WA, Allbritton EMA, McAdoo AG, Cannon-Smith DJ, Funwie NL, Hoang T, et al. Earth Abundant Oxidation Catalysts for Removal of Contaminants of Emerging Concern from Wastewater: Homogeneous Catalytic Screening of Monomeric Complexes. Molecules. 2023; 28(18):6466. https://doi.org/10.3390/molecules28186466
Chicago/Turabian StyleGarcia, Leslie, Makynna R. Koper, Somrita Mondal, Joshua T. Priddle, William A. Truong, Elisabeth M. A. Allbritton, Ashtyn G. McAdoo, Desiray J. Cannon-Smith, Neil L. Funwie, Tuyet Hoang, and et al. 2023. "Earth Abundant Oxidation Catalysts for Removal of Contaminants of Emerging Concern from Wastewater: Homogeneous Catalytic Screening of Monomeric Complexes" Molecules 28, no. 18: 6466. https://doi.org/10.3390/molecules28186466